Kv1.7
Description: potassium voltage-gated channel, shaker-related subfamily, member 7 Gene: Kcna7 Alias: Kv1.7, kcna7, HAK6
Kv1.7, encoded by the gene KCNA7, is a member of the potassium voltage-gated channel subfamily A. Kv1.7 plays a central role in cardiac atria repolarization [386]
Experimental data
Rat Kv1.7 gene in CHO host cells datasheet |
||
|
Click for details 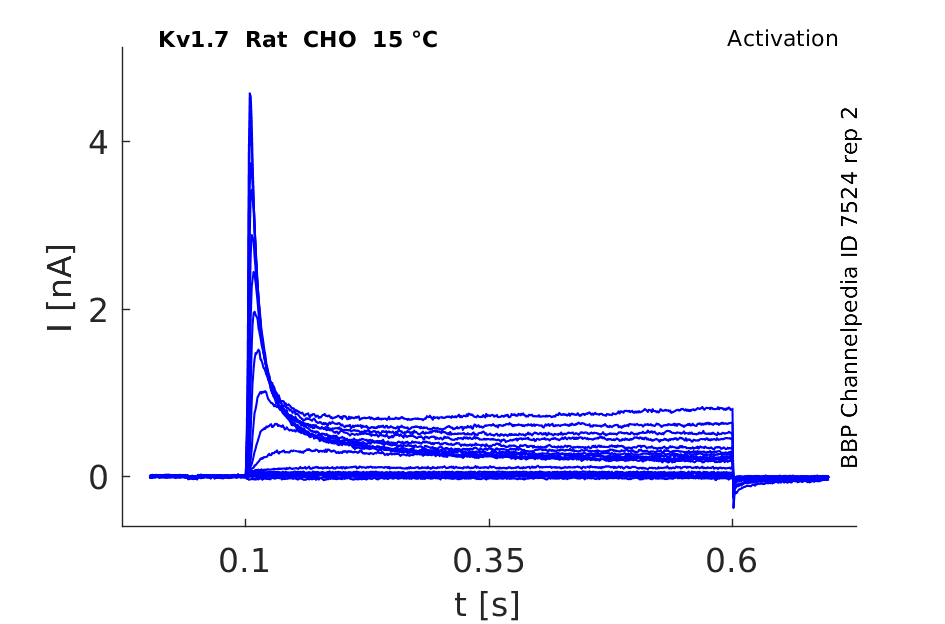
15 °Cshow 123 cells |
Click for details 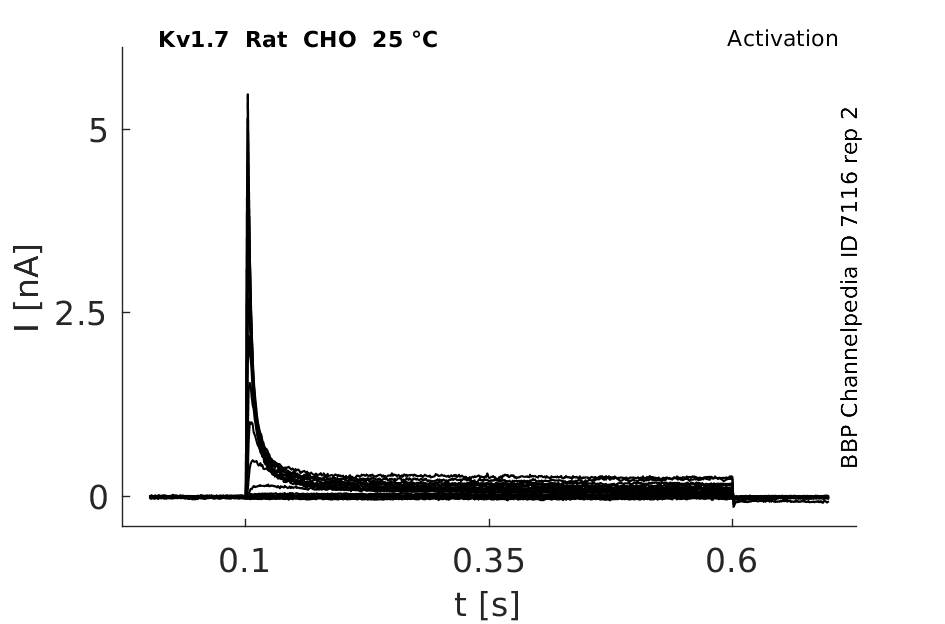
25 °Cshow 124 cells |
Click for details 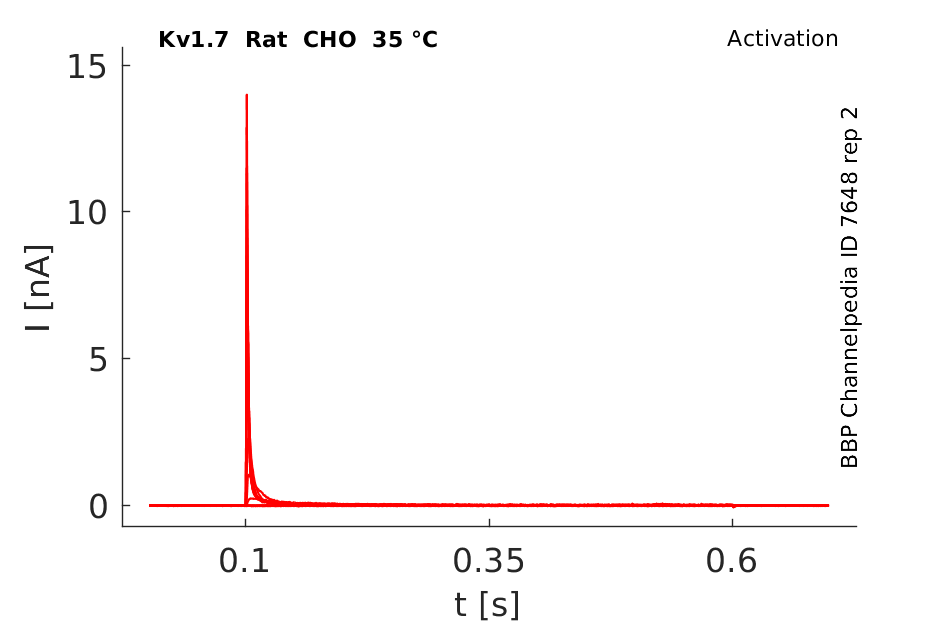
35 °Cshow 158 cells |
The murine Kv1.7 channels are encoded by the Kcna7 gene, composed of two exons separated by a 1.9-kb intron, and it is located in the mouse chromosome 7 [388].
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_031886.3 | 4151 | |
| Mouse | NM_010596.2 | 3494 | |
| Rat | NM_001108914.1 | 3592 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv1.7 Structure
Methodology for visual representation of structure available here
The N-terminal region of the Kv channels plays important regulatory roles, including inactivation kinetics [593], [594], [595], [557], [596], [597], subunit recognition [598], [599] and redox modulation of the currents flowing through those channels [600], [601]. Hence, the differences among N-terminal regions of Kv channels can result in important functional differences between the different molecular forms of Kv channels. [386].
Kv1.7 Antibody Mechanism of Action

STRUCTURE of KV1.7
The deduced mKv1.7 protein consists of 532 amino acids and contains six putative membrane-spanning domains, S1–S6. The hydrophobic core of this protein shares considerable sequence similarity with otherShaker family channels, while the intracellular N and C termini and the external loops between S1/S2 and S3/S4 show little conservation. The protein contains conserved sites for post-translational modifications. As do all other Shaker-related channels, mKv1.7 has a potential tyrosine kinase phosphorylation site (RPSFDAVLY) in its N-terminal region; the proline-rich stretch within the N terminus may be a binding site for SH3 domains of tyrosine kinases. Two protein kinase C consensus sites (Ser/Thr-X-Arg/Lys) are present in the cytoplasmic loop between S4 and S5 of mKv1.7; at least one of these sites is present in all members of the Kv1 family. mKv1.7, like Kv1.6, lacks anN-glycosylation site in the extracellular loop linking the S1 and S2 transmembrane segments; this consensus sequence is conserved in all other Kv1 family genes [388]
Kv1.7 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Kv1.7 Kinetics

Kinetics in Mouse Heart Muscle
Kv1.7 channels from mouse heart muscle have two putative translation initiation start sites that generate two channel isoforms with different functional characteristics, mKv1.7L (489 aa) and a shorter mKv1.7S (457 aa). The electrophysiological analysis of mKv1.7L and mKv1.7S channels revealed that the two channel isoforms have different inactivation kinetics. The channel resulting from the longer protein (L) inactivates faster than the shorter channels (S). Our data supports the hypothesis that mKv1.7L channels inactivate predominantly due to an N-type related mechanism, which is impaired in the mKv1.7S form. [386]
Single Channel Current
The mouse Kv1.7 channel is voltage-dependent and rapidly inactivating, exhibits cumulative inactivation, and has a single channel conductance of 21 pS. In an outside-out patch by applying 450-ms voltage ramps from −90 to 80 mV every second, single channel events were seen at potentials more positive than ∼−15 mV [388]

Kv1.7 currents in tsA-201 cells
Whole-cell current traces were recorded in tsA-201 cells expressing hKv1.7 channels. The currents were evoked by depolarization to 0 mV or to 40mV from a holding voltage of -80mV. Currents facilitated by the human homologue of Kv1.7. resembled those of the mouse short isoform of Kv1.7. [1650]
EXPRESSION IN HUMAN TISSUE
Transcripts of the Kcna7 gene are reported present in several tissues, including strong expression in the heart and skeletal muscle [386] Kv1.7 is also expressed in human the human kidney [1651]
EXPRESSION IN MOUSE
Kcna7 transcripts have been found in the mouse mesenteric artery [602] as well as in the rat main and small pulmonary arteries [603]
A Northern blot of poly(A)+ RNA from mouse heart, brain, spleen, lung, liver, skeletal muscle, kidney, and testis was probed with the mouse Kv1.7-specific 3′-noncoding region sequence [388]
Nephron: Kv1.7 in podocytes
Members of the Kv1 channel family display specific expression patterns in the nephron. Expression of Kv1.7 channels was reported in podocytes [2046]
Beta cells
Kv1.7 channels in the membrane of beta cells plays a role in the repetitive electrical spiking activity needed for glucose-stimulated insulin secretion (GSIS). Kv 1.7 channel contribute to the repolarizing current of beta cells during GSIS [1650]
Neuronal function
mRNAs for Kv1.7 and Kv3.4 are highly abundant in both the atrium and ventricle, which might indicate a functional role of these ion channel subunits in the formation of action potential in the human ventricle and both in the atrium and ventricle, respectively [1652]
Type 2 Diabetes
Conk-S1 increases GSIS in isolated rat islets, likely by reducing Kv1.7-mediated delayed rectifier currents in beta cells, which yields increases in action potential firing and cytoplasmic free calcium. In rats, Conk-S1 increases glucose-dependent insulin secretion without decreasing basal glucose. Thus, we conclude that Kv1.7 contributes to the membrane-repolarizing current of beta cells during GSIS and that block of this specific component of beta cell Kv current offers a potential strategy for enhancing GSIS with minimal risk of hypoglycaemia during metabolic disorders such as Type 2 diabetes [1650]
Cardiac Channel
Characterisation of the human voltage-gated potassium channel gene, KCNA7, a candidate gene for inherited cardiac disorders, and its exclusion as cause of progressive familial heart block I (PFHBI) [387]
(Conk-S1),
a naturally occurring cone-snail peptide toxin, Conkunitzin-S1, blocks Kv1.7 channels to provide an intrinsically limited, finely graded control of total beta cell delayed rectifier current and hence of glucose stimulated insulin secretion (GSIS) : 100% washout [1650]
ShK Toxin/Noxiustoxin/Margatoxin
The mKv1.7 channel is also potently blocked by a peptide (ShK toxin) obtained from sea anemone Stichodactyla helianthus(IC50 = 13 nM), and by the scorpion toxins, noxiustoxin (IC50 = 18 nM) and margatoxin (IC50 = 116 nM) [388]
TEA
Kv1.7 is insensitive to tetraethylammonium (because residue is hydrophobic) [388]
charybdotoxin/kaliotoxin
The channel was resistant to charybdotoxin (IC50 >1000 nM) and kaliotoxin (IC50 >1000 nM) [388]
Pharmacology
Pharmacological sensitivity of the mKv1.7 channel were performed, IC50 values in each case being determined when block reached steady-state. The channel was blocked by several non-peptide small molecule antagonists, 4-aminopyridine (IC50 = 245 μM), capsaicin (25 μM), cromakalim (450 μM), tedisamil (18 μM), nifedipine (13 μM), diltiazem (65 μM), and resiniferatoxin (20 μM). Surprisingly, the dihydroquinoline compound, CP-339,818, that blocks rapidly inactivating Kv1 channels in the nanomolar range (30), had little effect on mKv1.7 (IC50 = 10 μM) [388]
Anti-arythmic drugs
amiodarone (Kd=35±muM), flecainide (Kd=8±muM) and quinidine (Kd=15±2 muM). all block kv1.7 [387]
References
Finol-Urdaneta RK
et al.
Molecular and Functional Differences between Heart mKv1.7 Channel Isoforms.
J. Gen. Physiol.,
2006
Jul
, 128 (133-45).
Bardien-Kruger S
et al.
Characterisation of the human voltage-gated potassium channel gene, KCNA7, a candidate gene for inherited cardiac disorders, and its exclusion as cause of progressive familial heart block I (PFHBI).
Eur. J. Hum. Genet.,
2002
Jan
, 10 (36-43).
Kálmán K
et al.
Genomic organization, chromosomal localization, tissue distribution, and biophysical characterization of a novel mammalian Shaker-related voltage-gated potassium channel, Kv1.7.
J. Biol. Chem.,
1998
Mar
6
, 273 (5851-7).
Kashuba VI
et al.
Initial isolation and analysis of the human Kv1.7 (KCNA7) gene, a member of the voltage-gated potassium channel gene family.
Gene,
2001
May
2
, 268 (115-22).
MacKinnon R
et al.
Functional stoichiometry of Shaker potassium channel inactivation.
Science,
1993
Oct
29
, 262 (757-9).
MacDonald PE
et al.
Members of the Kv1 and Kv2 voltage-dependent K(+) channel families regulate insulin secretion.
Mol. Endocrinol.,
2001
Aug
, 15 (1423-35).
Nerbonne JM
Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium.
J. Physiol. (Lond.),
2000
Jun
1
, 525 Pt 2 (285-98).
Brown AM
Cardiac potassium channels in health and disease.
Trends Cardiovasc. Med.,
1997
May
, 7 (118-24).
Bezanilla F
et al.
Inactivation of the sodium channel. I. Sodium current experiments.
J. Gen. Physiol.,
1977
Nov
, 70 (549-66).
Hoshi T
et al.
Biophysical and molecular mechanisms of Shaker potassium channel inactivation.
Science,
1990
Oct
26
, 250 (533-8).
Zagotta WN
et al.
Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB.
Science,
1990
Oct
26
, 250 (568-71).
Roeper J
et al.
NIP domain prevents N-type inactivation in voltage-gated potassium channels.
Nature,
1998
Jan
22
, 391 (390-3).
Pongs O
et al.
Functional and molecular aspects of voltage-gated K+ channel beta subunits.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (344-55).
Li M
et al.
Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel.
Science,
1992
Aug
28
, 257 (1225-30).
Shen NV
et al.
Molecular recognition and assembly sequences involved in the subfamily-specific assembly of voltage-gated K+ channel subunit proteins.
Neuron,
1995
Mar
, 14 (625-33).
Ruppersberg JP
et al.
Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation.
Nature,
1991
Aug
22
, 352 (711-4).
Ciorba MA
et al.
Regulation of voltage-dependent K+ channels by methionine oxidation: effect of nitric oxide and vitamin C.
FEBS Lett.,
1999
Jan
8
, 442 (48-52).
Fountain SJ
et al.
Functional up-regulation of KCNA gene family expression in murine mesenteric resistance artery smooth muscle.
J. Physiol. (Lond.),
2004
Apr
1
, 556 (29-42).
Davies AR
et al.
Kv channel subunit expression in rat pulmonary arteries.
Lung,
2001
, 179 (147-61).
Finol-Urdaneta RK
et al.
Block of K(v) 1.7 potassium currents increases glucose-stimulated insulin secretion.
EMBO Mol Med,
2012
May
, 4 (424-34).
García-Villegas R
et al.
Potassium channels lost during harvesting of epithelial cells are restored with a kinetics that depends on channel species.
Cell. Physiol. Biochem.,
2007
, 20 (405-16).
Ordög B
et al.
Gene expression profiling of human cardiac potassium and sodium channels.
Int. J. Cardiol.,
2006
Aug
28
, 111 (386-93).
Carrisoza-Gaytán R
et al.
Differential expression of the Kv1 voltage-gated potassium channel family in the rat nephron.
J. Mol. Histol.,
2014
Oct
, 45 (583-97).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/7/ , accessed on 2026 Feb 22