Cav2.3
Description: calcium channel, voltage-dependent, R type, alpha 1E subunit Gene: Cacna1e Alias: cacna1e, cav2.3, ca2.3
Cav2.3, encoded by the gene cacna1e is calcium, voltage-gated, R type, alpha subunit channel. Cav2.3 is expressed ubiquitously but its predominant location is in the brain. It is involved in the generation of the R-type current and is responsible for the influx of Ca2+ allowing for neurotransmitter release.
In humans, cacna1e, the gene which encodes Cav2.3, is composed of 52 exons located on chromosome 1 at position 25. (1q25.3). [2427]
Like most Cav channels, the coding gene of Cav2.3, cacna1e, is subject to alternative splicing.
Several splice variants have been identified with vertebrates having at least 3 major splice events, generating mRNAs for Cav2.3c, Cav2.3d, and Cav2.3e. These have been shown to have significant differences in the amino terminus, II-III loop, and carboxy terminus [2464] [2465] [2466]
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_001205293.3 | 16420 | |
| Mouse | NM_009782.3 | 12697 | |
| Rat | NM_019294.3 | 7367 |
The human Cav2.3 protein is composed of 2313 amino acid (aa) and has a molecular weight of 261 Kda.
The CACNA1E gene undergoes extensive alternative splicing, resulting in Cav2.3 channels with different properties. The 3 major isoforms are Cav2.3c, Cav2.3d, and Cav2.3e [2464]
Isoforms
Like most mammalian proteins, Cav2.3 is subject to post translational modifications (PTM).
Cav2.3 is subject to phosphorylation with many phosphorylation sites having been identified, particularly within the II-III loop [2465] Loss of phosphorylation was shown to lead to aberrant function of the channel due to slower inactivation and enhanced cholinergic stimulation, causing in increased neuronal excitability. [2467]
There is also evidence of Cav2.3 glycosylation. Cryo-EM map of the channel highlighted a number of sites where glycosylations may occur [2468]
Like most Cav channels, Cav2.3 is made up of a single protein composed of 4 homologous domains (DI-DIV). Each domain is made up of 6 transmembrane subunits (S1-S6) connected by extracellular loops. S1-4 form the voltage sensing domain (VSD) whereas the S5-S6 act as the selectivity filter and form the pore module (PM). The S4 subunit of each domain contains a series of positively charged residues. The N-terminus and C-terminus of the α1 subunit have important roles in trafficking and anchoring of the subunit to the cell membrane. The intracellular loop between domains II and III of the α1 subunit contains a domain that can interact with G-protein-coupled receptors, as well as key motifs that interact with SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins, which are important for vesicular docking before exocytosis [2468]
The structure of human Cav2.3 in complex with the α2δ1 and β1 subunits was resolved via cryo-electron microscopy to an overall resolution of 3.1 angstroms, giving us a detailed insight to the channel’s architecture. Structural resolution of the ion channel highlighted a number of Cav2.3 specific features [2468]:
- Cav2.3’s resistance to several Cav modulators is due to structural differences. Notably, substitutions at positions Y1296 and F1708 obstruct ligand binding at the DIII-DIV fenestration, and M1300 reduces sensitivity to dihydropyridine molecules. Other structural differences in the first extracellular loop (ECL) also contribute to Cav2.3's resistance to ziconotide.
- The extracellular loop IV (ECLIV) in Cav2.3 is longer than in Cav2.2 and interacts with the S1-S2III linker, stabilizing the voltage-sensing domain III (VSDIII) and affecting the channel's activation properties. This interaction causes a positive shift in the voltage dependency of Cav2.3 activation
- The VSDII of Cav2.3 remains in the resting state, unlike other voltage-gated channels where it is typically in the activated state. This indicates a unique role of VSDII in Cav2.3 gating
Cav2.3 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Cav2.3 current is characterized by its activation at more negative potentials and its inactivation which occurs more rapidly and completely compared to other Cavs. In neocortical pyramidal cells, the half-activation voltage was significantly more negative than other high voltage activated current types in these cells. This was also the case in vitro experiments, with Cav2.3 expression in oocytes. [2469]
CaV2.3 channels also exhibit cumulative inactivation in response to brief and repetitive depolarizations. This process known as preferential closed-state inactivation (CSI) [2468]
The function consequences of these properties are that these hyperpolarized activation voltage range of Cav2.3 may facilitate a role in synaptic integration in the subthreshold voltage range in those cells. The hyperpolarized inactivation range and rapid inactivation, means that Cav2.3 is more sensitive to sustained depolarizations and thus may play a lesser role during sustained activity. Due to the more depolarized half-inactivation voltage in medium spiny neurons, R-type current in these cells may be less inactivated during sustained depolarizations. [2469]
Single Channel Unitary Conductance
There has been no single channel unitary conductance recordings for Cav2.3
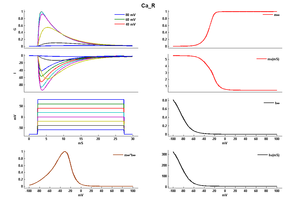
Model
Model Ca_R (ID=7)
| Animal | rat | |
| CellType | Cerebellar | |
| Age | 21 Days | |
| Temperature | 36.0°C | |
| Reversal | 135.0 mV | |
| Ion | Ca + | |
| Ligand ion | ||
| Reference | [261] T Miyasho et. al; Brain Res. 2001 Feb 9 | |
| mpower | 1.0 | |
| m Alpha | 2.6/(1+exp((v+7)/-8)) | |
| m Beta | 0.18/(1+exp((v+26)/4)) | |
| hpower | 1.0 | |
| h Alpha | 0.0025/(1+exp((v+32)/8)) | |
| h Beta | 0.19/(1+exp((v+42)/-10)) | |
Cav2.3 is expressed widely throughout both the central and peripheral nervous system
Within the CNS, Cav2.3 is widespread in the brain with higher levels in the hippocampus, interpeduncular nucleus, striatum, pallidum, cortex, amygdala, olfactory tubercle, accumbens, and dorsal cochlear nucleus than in other regions. [2470].
Cav2.3 has also been found consistently present in the endocrine, cardiovascular, gastrointestinal and reproductive systems in tissues such as the kidney, retina, spleen, and pancreatic islet cells. [2471] [2472] [2415]
With neuronal cells, Cav2.3 is predominantly expression in both pre-synaptic and post-synaptic locations in the dendrites. It can also be located at the cell soma [2415] [2472]
Exact subcellular distribution is dependent on the tissue or cell where the channel is expressed. Cav2.3 is predominantly presynaptic in the interpeduncular nucleus, but mainly expressed postsynaptically in other brain regions. [2470]
Cav2.3’s role is to mediate the entry of calcium into excitable cells. In its dominant expression locations, Cav2.3 generates large calcium spikes in response to both back-propagating action potentials and synaptic activity, thus enhancing Cav2.3 neuronal excitability in neuronal dendrites [2473]
Neurotransmitter release
The entry of calcium via Cav2.3 is also responsible for triggering neurotransmitter release at the synapse, though at a lower efficiency compared to Cav2.1 and Cav2.2 [2415] [521] [2474] [2464]
Long term potentiation & synaptic plasticity
Synpatic plasticity requires large amounts of Ca2+ influx for the initial step of long term potentiation inductions. In Cav2.1 and Cav2.2 blockage experiments, Cav2.3 was responsible for the accumulation of presynaptic Ca2+, highlighting its role in long term potentiation. [521] [2468]
Nociception
Cav2.3 is thought to contribute to pain pathways, particularly in the neuciceptive neurons at the sensory ganglia, where it is present. There has been evidence that Cav2.3 can be inhibited by analgesic drugs [2475]. Mice lacking Cav2.3 also showed altered pain responses, further highlight Cav2.3’s role in nociception [2465]
Secretion
Cav2.3 is thought to maybe has a role in insulin secretion. Despite knockout models not altering β-cell mass and insulin content directly, CaV2.3-deficient mice exhibited disturbances in glucose tolerance and insulin secretion as well as hyperglycemia. These results indicate that CaV2.3 channels may selectively control second phase insulin secretion. [2453]
SK channel
Cav2.3 mediated calcium influx was shown to selectively activate Sk channels, which was inhibited when Cav2.3 was blocked [2476]
Channelopathies
Given Cav2.3 roles in neuronal excitability and neurotransmission, dysfunction of the channel leads to a number of pathologies:
Neurodevelopmental disorders
The most prominent pathology associated with Cav2.3 is Fragile X syndrome (FXS). Fragile X syndrome (FXS) is an inherited intellectual impairment that results from the loss of fragile X mental retardation protein (FMRP), an mRNA binding protein that regulates mRNA translation at synapses. Patients with fragile X syndrome (FXS) exhibit signs of neuronal and circuit hyperexcitability, including anxiety and hyperactive behavior, attention deficit disorder, and seizures In the absence of FMRP, regulation fo the expression and activity of voltage-gated ion channels is altered, namely Cav2.3. Alterations in channel translation and expression observed in the absence of FMRP contribute to the neuronal hyperactivity that underlies FXS. [2473]
Other mutations to Cav2.3 are frequently associated with:
- Epilepsy [2477]
- Bipolar disorder [2478]
- Intellectual disability (ID)/global developmental delay (GDD) [2415]
- Autism spectrum disorder [2460]
Aberrant function of Cav2.3 is involved in the progression of Parkinson's. Cav2.3 is a key regulator in the nigral viability. The presence of Cav2.3 transcripts, which increases in abundance with age, was associated with the development of Parkingsons. In Cav2.3 KO mice models, Cav2.3 deficiency upregulated transcripts for NCS-1, a Ca2+-binding protein implicated in neuroprotection. Conversely, NCS-1 knockout exacerbated nigral neurodegeneration and downregulated Cav2.3.
CACNA1E, along with other Cav genes, was found under-expressed in breast cancer. However, this under expressing is probably a consequences of the disease and the channel is not the cause for such cancers [2440]
When it was first discovered, Cav2.3 was coined as “R-type” as it was resistant to multiple common Cav channel blockers (nimodipine, ω-CTX GVIA, and ω-Aga IVA). However, Cav2.3 is no longer “resistant”, as a non specific Cav blocker was identified SNX-482 [2453] [2415]
Auxiliary subunit
Cav2.3 channels typically exist as multi-subunit complexes composed of the main pore-forming α1 subunit, as previously described, and auxiliary subunits α2δ-, β-subunits. When coexpressed in Hek cells with Cav2.3, CaVα2δ2 and CaV2.3/β3 increased the current density CaV2.3/β3, suggesting enhanced surface expression of the channels. CaVα2δ3 also dramatically increased the current amplitude measured in cells expressing CaV2.3/β3 [2453]
CaM & Calcium dependent inactivation
Cav2.3 were demonstrated to undergo Ca(2+)-dependent inactivation, mediated by the binding of CaM to the N-terminal love of the protein . [2479]
Other interactions
The N-terminus of Cav2.3 is additionally involved in G-protein coupled channel modulation [2472] Stimulation of α1E is selectively blocked by regulator of G-protein signaling 2 (RGS2) and the C-terminal region of phospholipase C-β1 (PLCβ1ct), two proteins known to function as GTPase-activating proteins (GAPs) for Gαq. [2464] Cav2.3 is both inhibited and stimulated through G-alpha-q/11-coupled muscarinic acetylcholine receptors [526], [89].
Cav2.3 channels are very sensitive towards divalent metal cations [2465] This was first seen in vitro, with Cav2.3 being identified as modulated by Zn2+ and Cu2+. In vivo, Cav2.3 is thought to be under tight allosteric control by endogenous loosely bound trace metal cations (Zn2+ and Cu2+) that suppress channel gating via a high-affinity trace–metal-binding site. [2480]
Interaction with alpha1C channel: A chimeric channel containing the I-II linker from alpha1E (Cav2.3) accelerated the inactivation kinetics of alpha1C [527].
Mibefradil (RO 40-5967), which has been used clinically as an anti-hypertensive and antianginal agent is modulating Ca2.3's inactivation kinetics. [100]
References
Meza U
et al.
Neurokinin 1 receptors trigger overlapping stimulation and inhibition of CaV2.3 (R-type) calcium channels.
Mol. Pharmacol.,
2007
Jan
, 71 (284-93).
Berrou L
et al.
Molecular determinants of inactivation within the I-II linker of alpha1E (CaV2.3) calcium channels.
Biophys. J.,
2001
Jan
, 80 (215-28).
Pérez-Alvarez A
et al.
Pharmacological and biophysical properties of Ca2+ channels and subtype distributions in human adrenal chromaffin cells.
Pflugers Arch.,
2008
Sep
, 456 (1149-62).
Raybaud A
et al.
The role of distal S6 hydrophobic residues in the voltage-dependent gating of CaV2.3 channels.
J. Biol. Chem.,
2007
Sep
21
, 282 (27944-52).
Bernatchez G
et al.
State-dependent inhibition of inactivation-deficient Ca(V)1.2 and Ca(V)2.3 channels by mibefradil.
J. Membr. Biol.,
2001
Nov
15
, 184 (143-59).
Cohen R
et al.
The C2A domain of synaptotagmin alters the kinetics of voltage-gated Ca2+ channels Ca(v)1.2 (Lc-type) and Ca(v)2.3 (R-type).
J. Biol. Chem.,
2003
Mar
14
, 278 (9258-66).
Berrou L
et al.
A specific tryptophan in the I-II linker is a key determinant of beta-subunit binding and modulation in Ca(V)2.3 calcium channels.
Biophys. J.,
2002
Sep
, 83 (1429-42).
Miyasho T
et al.
Low-threshold potassium channels and a low-threshold calcium channel regulate Ca2+ spike firing in the dendrites of cerebellar Purkinje neurons: a modeling study.
Brain Res.,
2001
Feb
9
, 891 (106-15).
Randall AD
et al.
Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels.
Neuropharmacology,
1997
Jul
, 36 (879-93).
Zhang JF
et al.
Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons.
Neuropharmacology,
1993
Nov
, 32 (1075-88).
Newcomb R
et al.
Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas.
Biochemistry,
1998
Nov
3
, 37 (15353-62).
Wu L
et al.
Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2.
Nature,
2002
Oct
31
, 419 (947-52).
Kuzmiski JB
et al.
Topiramate inhibits the initiation of plateau potentials in CA1 neurons by depressing R-type calcium channels.
Epilepsia,
2005
Apr
, 46 (481-9).
Metz AE
et al.
R-type calcium channels contribute to afterdepolarization and bursting in hippocampal CA1 pyramidal neurons.
J. Neurosci.,
2005
Jun
15
, 25 (5763-73).
Tai C
et al.
Muscarinic enhancement of R-type calcium currents in hippocampal CA1 pyramidal neurons.
J. Neurosci.,
2006
Jun
7
, 26 (6249-58).
Sabatini BL
et al.
Analysis of calcium channels in single spines using optical fluctuation analysis.
Nature,
2000
Nov
30
, 408 (589-93).
Kubota M
et al.
Intact LTP and fear memory but impaired spatial memory in mice lacking Ca(v)2.3 (alpha(IE)) channel.
Biochem. Biophys. Res. Commun.,
2001
Mar
23
, 282 (242-8).
Breustedt J
et al.
Alpha1E-containing Ca2+ channels are involved in synaptic plasticity.
Proc. Natl. Acad. Sci. U.S.A.,
2003
Oct
14
, 100 (12450-5).
Dietrich D
et al.
Functional specialization of presynaptic Cav2.3 Ca2+ channels.
Neuron,
2003
Jul
31
, 39 (483-96).
Yokoyama K
et al.
Blocking the R-type (Cav2.3) Ca2+ channel enhanced morphine analgesia and reduced morphine tolerance.
Eur. J. Neurosci.,
2004
Dec
, 20 (3516-9).
Osanai M
et al.
Altered cerebellar function in mice lacking CaV2.3 Ca2+ channel.
Biochem. Biophys. Res. Commun.,
2006
Jun
9
, 344 (920-5).
Saegusa H
et al.
Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel.
Proc. Natl. Acad. Sci. U.S.A.,
2000
May
23
, 97 (6132-7).
Murakami M
et al.
Antinociceptive effect of different types of calcium channel inhibitors and the distribution of various calcium channel alpha 1 subunits in the dorsal horn of spinal cord in mice.
Brain Res.,
2004
Oct
22
, 1024 (122-9).
Meza U
et al.
Biphasic, opposing modulation of cloned neuronal alpha1E Ca channels by distinct signaling pathways coupled to M2 muscarinic acetylcholine receptors.
J. Neurosci.,
1999
Aug
15
, 19 (6806-17).
Stotz SC
et al.
Fast inactivation of voltage-dependent calcium channels. A hinged-lid mechanism?
J. Biol. Chem.,
2000
Aug
11
, 275 (24575-82).
Kessi M
et al.
Calcium channelopathies and intellectual disability: a systematic review.
Orphanet J Rare Dis, 2021May13, 16 (219).
Rajakulendran S
et al.
Neuronal P/Q-type calcium channel dysfunction in inherited disorders of the CNS.
,
2012
Jan
17
, ().
Phan NN
et al.
Voltage-gated calcium channels: Novel targets for cancer therapy.
Oncol Lett, 2017Aug, 14 (2059-2074).
Yang SN
et al.
The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology.
Endocr. Rev.,
2006
Oct
, 27 (621-76).
Liao X
et al.
Genetic associations between voltage-gated calcium channels and autism spectrum disorder: a systematic review.
Mol Brain, 2020Jun22, 13 (96).
Melliti K
et al.
Muscarinic stimulation of alpha1E Ca channels is selectively blocked by the effector antagonist function of RGS2 and phospholipase C-beta1.
J. Neurosci.,
2000
Oct
1
, 20 (7167-73).
Schneider T
et al.
In vitro and in vivo phosphorylation of the Cav2.3 voltage-gated R-type calcium channel.
Channels (Austin), 2018, 12 (326-334).
Pereverzev A
et al.
Structural diversity of the voltage-dependent Ca2+ channel alpha1E-subunit.
Eur. J. Neurosci.,
1998
Mar
, 10 (916-25).
Sampedro-Castañeda M
et al.
Epilepsy-linked kinase CDKL5 phosphorylates voltage-gated calcium channel Cav2.3, altering inactivation kinetics and neuronal excitability.
Nat Commun, 2023Dec11, 14 (7830).
Gao Y
et al.
Molecular insights into the gating mechanisms of voltage-gated calcium channel CaV2.3.
Nat Commun, 2023Jan31, 14 (516).
Foehring RC
et al.
Unique properties of R-type calcium currents in neocortical and neostriatal neurons.
J. Neurophysiol.,
2000
Nov
, 84 (2225-36).
Parajuli LK
et al.
Quantitative regional and ultrastructural localization of the Ca(v)2.3 subunit of R-type calcium channel in mouse brain.
J. Neurosci.,
2012
Sep
26
, 32 (13555-67).
Neumaier F
et al.
Cav2.3 channel function and Zn2+-induced modulation: potential mechanisms and (patho)physiological relevance.
Channels (Austin), 2020Dec, 14 (362-379).
Weiergräber M
et al.
The Ca(v)2.3 voltage-gated calcium channel in epileptogenesis--shedding new light on an enigmatic channel.
,
2006
, 30 (1122-44).
Gray EE
et al.
Disruption of GpI mGluR-Dependent Cav2.3 Translation in a Mouse Model of Fragile X Syndrome.
J Neurosci, 2019Sep18, 39 (7453-7464).
de Amorim Ferreira M
et al.
Role of Cav2.3 (R-type) Calcium Channel in Pain and Analgesia: A Scoping Review.
Curr Neuropharmacol, 2024, 22 (1909-1922).
Bloodgood BL
et al.
Nonlinear regulation of unitary synaptic signals by CaV(2.3) voltage-sensitive calcium channels located in dendritic spines.
Neuron,
2007
Jan
18
, 53 (249-60).
Heyne HO
et al.
De novo variants in neurodevelopmental disorders with epilepsy.
Nat Genet, 2018Jul, 50 (1048-1053).
Jan WC
et al.
Exploring the associations between genetic variants in genes encoding for subunits of calcium channel and subtypes of bipolar disorder.
J Affect Disord,
2014
Mar
, 157 (80-6).
Liang H
et al.
Unified mechanisms of Ca2+ regulation across the Ca2+ channel family.
Neuron,
2003
Sep
11
, 39 (951-60).
Schneider T
et al.
Cav2.3 R-type calcium channels: from its discovery to pathogenic de novo CACNA1E variants: a historical perspective.
Pflugers Arch, 2020Jul, 472 (811-816).
Contributors: Rajnish Ranjan, Michael Schartner
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/82/ , accessed on 2026 Feb 24