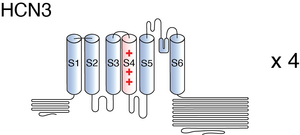
HCN3
Description: hyperpolarization-activated cyclic nucleotide-gated potassium channel 3 Gene: Hcn3 Alias: HCN3, Hac3, Hcn3, Bcng4, BCNG-4, ih3
HCN3, encoded by the gene hcn3, is a hyperpolarization-activated cyclic nucleotide-gated potassium channel. HCN3 is widely expressed at low levels throughout both the CNS and PNS. It is involved in the generation of the I(h) current which controls neuron excitability.
Experimental data
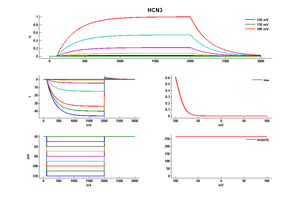
Rat HCN3 gene in CHO host cells |
||
|
Click for details 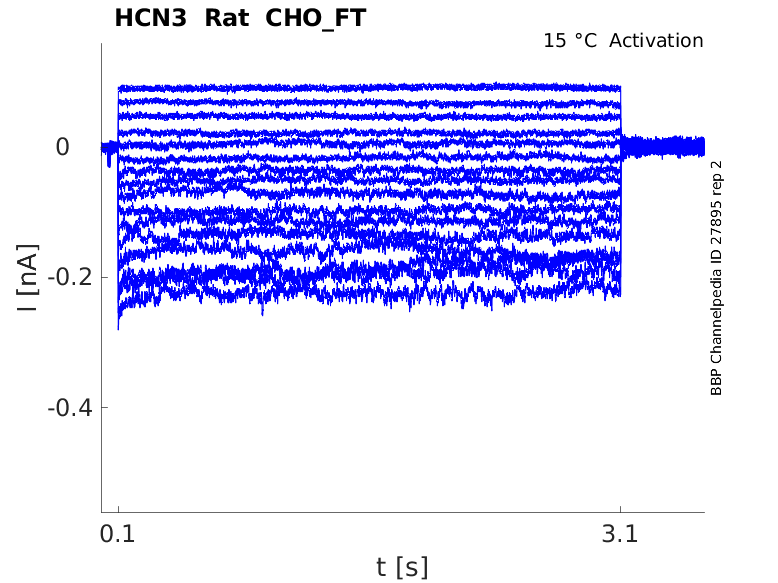
15 °Cshow 16 cells |
Click for details 
25 °Cshow 58 cells |
Click for details 
35 °Cshow 95 cells |
Mouse HCN3 gene in CHO host cells |
||
|
Click for details 
15 °Cshow 29 cells |
Click for details 
25 °Cshow 53 cells |
Click for details 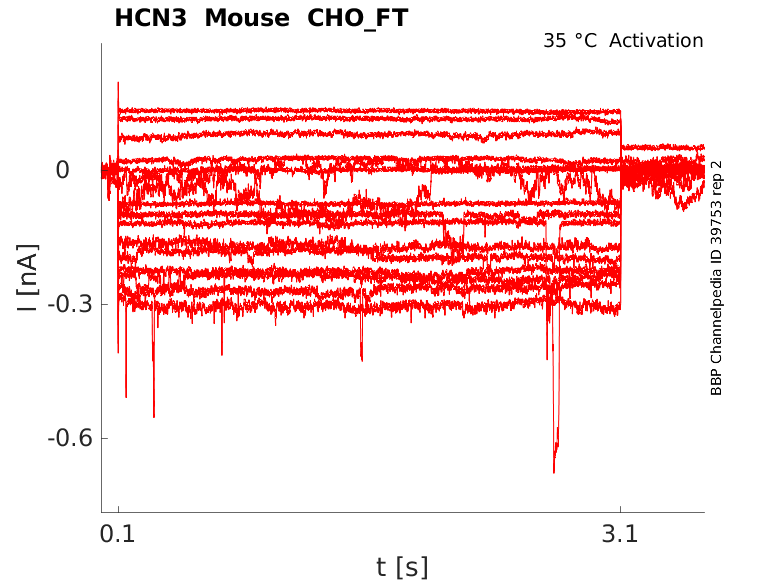
35 °Cshow 21 cells |
Human HCN3 gene in CHO host cells |
||
|
Click for details 
15 °Cshow 17 cells |
Click for details 
25 °Cshow 36 cells |
Click for details 
35 °Cshow 13 cells |
hcn3 is the coding gene for HCN3. In humans, hcn3 is located on chromosome 1q22 and is made up of 8 exons, all of which are coding.
A splice variant of HCN3 has been identified in the liver and kidney, although the functional importance of this transcript remains to be established. [2340]
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_020897.3 | 3838 | |
| Mouse | NM_008227.1 | 3268 | |
| Rat | NM_053685.1 | 2343 |
The human HCN3 protein is composed of 774 amino acids (aa) and has a molecular weight of 86 Kda.
Isoforms
To date, no PTMs, specific to HCN3, have been studied.
Visual Representation of HCN3 Structure
Methodology for visual representation of structure available here
Human HCN3 is the smallest member within the human HCN protein family, primarily due to distinct variations in the lengths of its N- and C-terminal domains. Specifically, the N-terminal domain of hHCN3 consists of 88 amino acids, while the C-terminal domain comprises 220 amino acids. In contrast, the corresponding domains in hHCN4 extend to 257 and 481 amino acids, respectively. As a consequence of these variations, HCN3 shares an overall sequence homology of approximately 46-56% with other hHCNs. Nevertheless, the core region of HCN3, encompassing the six transmembrane segments, the pore region, the C-linker, and the cyclic nucleotide-binding domain (CNBD), exhibits a high degree of conservation across all hHCNs. [56].
HCN3 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
HCN3 exhibits relatively slow activation kinetics, characterized by time constants spanning from 250 to 400 milliseconds at a membrane potential of -140 mV. These activation kinetics are comparatively slower than those of HCN1 and HCN2 but faster than the corresponding kinetics of HCN4. The midpoint of activation (V1/2) for HCN3 falls within the range of -80 to -95 mV, and its reversal potential (Erev) has been determined to be approximately -20.5 +/- 4 mV. Consequently, HCN3 demonstrates a permeability ratio (PNa/Pk) of 0.3. [56] [457]
Single channel unitary conductance
There has been no single channel unitary conductance recordings for HCN3.
Model
Model HCN3 (ID=11)
| Animal | Mouse | |
| CellType | Dorsal root ganglion | |
| Age | 21 Days | |
| Temperature | 0.0°C | |
| Reversal | -45.0 mV | |
| Ion | Hcn + | |
| Ligand ion | ||
| Reference | [57] S Moosmang et. al; Eur. J. Biochem. 2001 Mar | |
| mpower | 1.0 | |
| m Inf | 1.0000/(1+exp((v- -96)/8.6)) | |
| m Tau | 265.0000 | |
Cellular and tissue
In the CNS, HCN3 is expressed at very low levels throughout the brain, with a few expression hotspots that appear to vary across species [338]. In humans, these hotspots were identified as the cerebellum, the olfactory bulb, and some hypothalamic nuclei [337] [2306] [457].
In the PNS, HCN3 has been identified in 60% of small (<20 μm diameter) and medium–large (>20 μm diameter) neuron subtypes. In the DRG neurons, HCN3 was found expressed in about 35% of small unmyelinated neurons, 86% of large myelinated neurons, and 63% of small and medium neurons with nociceptive function [2342]. HCN3 was also detected in heart muscles, albeit at relatively low levels [457].
For a more detailed map immunohistochemical localisation of HCN3, please consult the following paper [323]
Developmental
The highest expression level of HCN3 was found to be in the fetal brain. This notable finding was universal across most species, pointing to a potential role of HCN3 in early brain development. [56] [2306]
To date, the subcellular location of HCN3 has not been identified in the CNS.
HCN3 is responsible for generating the I(h) current in the tissues where it is present. In particular, studies have demonstrated that HCN3 plays a pivotal role in maintaining excitability and promoting rhythmic burst firing within hypothalamic nuclei. [2341]
Given its location in nociceptive neurons, HCN3 is thought to contribute to their intrinsic excitability. HCN3 deletion experiments have revealed varying effects: small neuron firing was minimally affected, while the firing of medium-sized neurons was enhanced. However, it is worth noting that HCN3 knockout mice primarily exhibited increased mechanical hyperalgesia, with levels of mechanical allodynia and thermal hyperalgesia remaining comparable to those in wild-type mice. Despite its presence in PNS neurons and its role in modulating their excitability, HCN3 does not appear to play a prominent role in the processing of acute, neuropathic, or inflammatory pain. [2342] [2318]
Cardiomyocytes of HCN3-deficient mice exhibited a notable reduction of approximately 30% in the total I(h) current. Interestingly, these mice lacking HCN3 did not manifest any pathological phenotypes and remained viable and in good health. These results suggest that while HCN3 may not be indispensable, it is likely to exert a significant influence on the configuration of the cardiac action potential waveform. [2342] [2343]
HCN3 is thought to also contribute to the coordination of proximal-to-distal peristalsis of the upper urinary tract. [2344]
Channelopathies
Single gene diseases associated with HCN3 have not been yet identified. However, HCN3 expression has been found upregulated in certain neuroblastoma tissue. Mutations in HCN3 were also identified in certain cases of epilepsy [2319].
cAMP & cGMP
Despite having a cAMP-binding domain, HCN3 is largely insensitive to both cAMP and cGMP [2318]. cAMP and cGMP have no significant impact on activation kinetics but do induce a 5 mV shift of the half-maximal activation voltage (V1/2) to more hyperpolarized potentials [55]. This lack of sensitivity of HCN3, despite harboring a functional CNBD, has been explained by its shorter C-terminal sequence, which alters the normal autoinhibition of the channel [2293].
PIP(2)
As with all HCNs, intracellular phosphatidylinositol-4,5-bisphosphate (PIP(2)) shifts HCN3 activation to more depolarized potentials and accelerated activation kinetics [2341].
For further compounds interactions, please consult the following resource
References
Mistrik P
et al.
The murine HCN3 gene encodes a hyperpolarization-activated cation channel with slow kinetics and unique response to cyclic nucleotides.
J. Biol. Chem.,
2005
Jul
22
, 280 (27056-61).
Stieber J
et al.
Functional expression of the human HCN3 channel.
J. Biol. Chem.,
2005
Oct
14
, 280 (34635-43).
Moosmang S
et al.
Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues.
Eur. J. Biochem.,
2001
Mar
, 268 (1646-52).
Notomi T
et al.
Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain.
J. Comp. Neurol.,
2004
Apr
5
, 471 (241-76).
Monteggia LM
et al.
Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain.
Brain Res. Mol. Brain Res.,
2000
Sep
30
, 81 (129-39).
Moosmang S
et al.
Differential distribution of four hyperpolarization-activated cation channels in mouse brain.
Biol. Chem.,
1999 Jul-Aug
, 380 (975-80).
Biel M
et al.
Hyperpolarization-activated cation channels: from genes to function.
Physiol. Rev.,
2009
Jul
, 89 (847-85).
Sartiani L
et al.
The Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels: from Biophysics to Pharmacology of a Unique Family of Ion Channels.
Pharmacol Rev, 2017Oct, 69 (354-395).
Santoro B
et al.
Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels as Drug Targets for Neurological Disorders.
Annu Rev Pharmacol Toxicol, 2020Jan06, 60 (109-131).
Lainez S
et al.
HCN3 ion channels: roles in sensory neuronal excitability and pain.
J Physiol, 2019Sep, 597 (4661-4675).
Kessi M
et al.
The Contribution of HCN Channelopathies in Different Epileptic Syndromes, Mechanisms, Modulators, and Potential Treatment Targets: A Systematic Review.
Front Mol Neurosci, 2022, 15 (807202).
DiFrancesco D
The role of the funny current in pacemaker activity.
Circ. Res.,
2010
Feb
19
, 106 (434-46).
Santoro B
et al.
The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (741-64).
Ying SW
et al.
PIP2-mediated HCN3 channel gating is crucial for rhythmic burst firing in thalamic intergeniculate leaflet neurons.
J. Neurosci.,
2011
Jul
13
, 31 (10412-23).
Dewar MB
HCN3 has minimal involvement in the sensation of acute, inflammatory and neuropathic pain.
J Physiol, 2019Nov, 597 (5333-5334).
Fenske S
et al.
HCN3 contributes to the ventricular action potential waveform in the murine heart.
Circ. Res.,
2011
Oct
14
, 109 (1015-23).
Hurtado R
et al.
The pelvis-kidney junction contains HCN3, a hyperpolarization-activated cation channel that triggers ureter peristalsis.
Kidney Int.,
2010
Mar
, 77 (500-8).
Contributors: Katherine Johnston, Rajnish Ranjan, Michael Schartner, Nitin Khanna
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/63/ , accessed on 2026 Feb 25