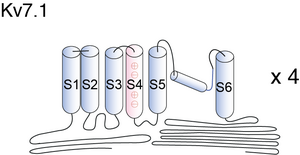
Kv7.1
Description: potassium voltage-gated channel, KQT-like subfamily, member 1 Gene: Kcnq1 Alias: KV7.1, LQT, RWS, WRS, LQT1, SQT2, ATFB1, ATFB3, JLNS1, KCNA8, KCNA9, Kv1.9, KVLQT1, KCNQ1
Kv7.1, encoded by the gene KCNQ1, is a member of the potassium voltage-gated channel KQT-like subfamily. These channels, which transport positively charged atoms (ions) of potassium into and out of cells, play a key role in a cell's ability to generate and transmit electrical signals[248] Kv7.1, is also known as: LQT; RWS; WRS; LQT1; SQT2; ATFB1; ATFB3; JLNS1; KCNA8; KCNA9; KVLQT1; FLJ26167.
Experimental data
Rat Kv7.1 gene in CHO host cells datasheet |
||
|
Click for details 
15 °Cshow 104 cells |
Click for details 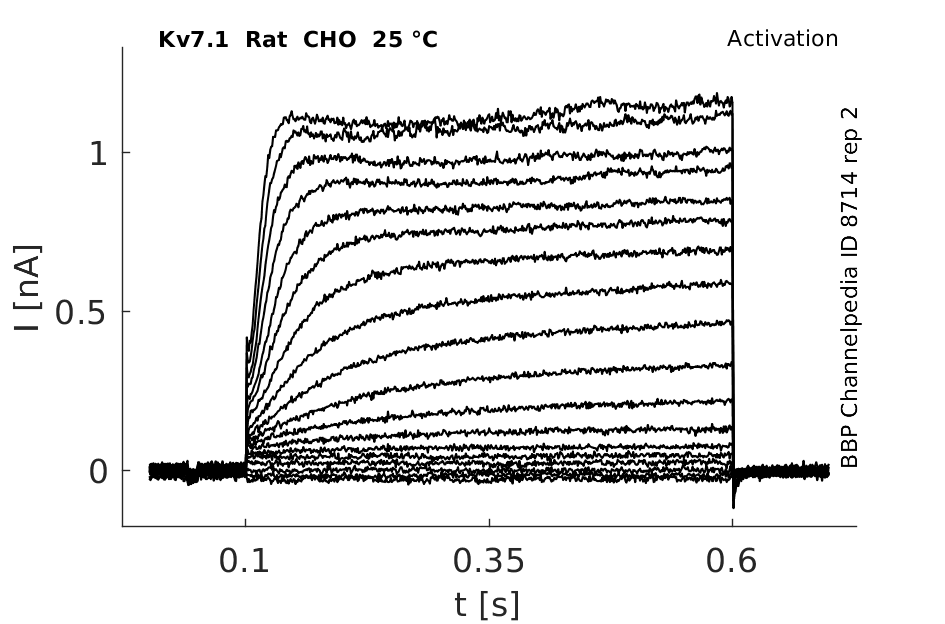
25 °Cshow 45 cells |
Click for details 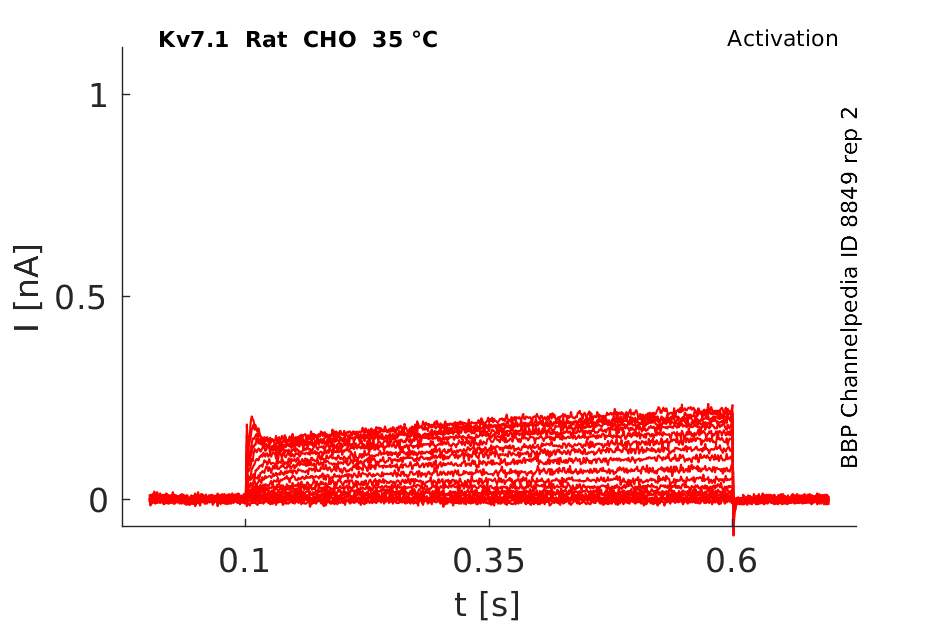
35 °Cshow 88 cells |
The gene is located in a region of chromosome 11 that contains a number of contiguous genes, which are abnormally imprinted in cancer and the Beckwith-Wiedemann syndrome. This gene is also imprinted, with preferential expression from the maternal allele in some tissues, excluding cardiac muscle. Alternatively spliced transcripts encoding distinct isoforms have been described. NCBI
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_000218.3 | 3224 | |
| Mouse | NM_008434.2 | 3052 | |
| Rat | NM_032073.2 | 3017 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv7.1 Structure
Methodology for visual representation of structure available here
KV7.1 STRUCTURE
KCNQ1, previously named KvLQT1, was first identified in a linkage study looking at some of the genetic causes of sudden death from cardiac arrhythmia. To date, five genes of this family (KCNQ1-5), all encoding K+ channel subunits with the ‘‘shaker-like’’ motif of 6TMD and a single P-loop, have been identified. The proteins share between 30 and 65% amino acid identity, with particularly high homology in the transmembrane regions. The S4 TMD, as other shaker- like K + channels, has been suggested to form the voltage sensor. A regular distribution of positively charged amino acids is seen. All KCNQ subunits have a regular distribution of six positively charged amino acids in the S4 region, except KCNQ1, which has four. The P- loop contains the K+ pore signature sequence TxxTxGYG. All five proteins display a highly homolo- gous region on their intracellular C-terminus termed the ‘‘A-domain’’ [722]
The α-subunit of KvLQT1 has the domain structure composed of six membrane-spanning segments (S1–S6) containing a pore region localized at cell membrane and the N-terminal and C-terminal regions that are localized in the cytoplasm. The KvLQT1 channel consists of four α-subunits [688] and two β-subunits (MinK) encoded by KCNE1 [689], which generates slow component of delayed rectifier potassium current (IKs).
Crystal Structure of Open and CLosed State

CRYSTAL STRUCTURE
Top-down (extracellular) view of the all atom system. Each of the 4 channel subunits (consisting of S1–S6 segments) is color coded. Pore regions (S5–S6) of one subunit interact with the adjacent subunit voltage-sensing region (S1–S4). (C) Voltage-sensing region with lipid of the 4 channel subunits (consisting of S1–S6 segments) (gray) and water (light blue) solvent molecules.[1676]

Kv7.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
KV7.1 kinetics

Oocytes injected with KVLQT1 cRNA exhibited robust outward currents that activated at potentials positive to −60 mV and exceeded 5 μA at +40 mV (less than 150 nA at +40 mV in the water-injected oocytes). KvLQT1 currents exhibited a delayed rectifier current phenotype and rectified weakly at positive voltages. Tail currents, elicited upon repolarization to −80 mV, exhibited an initial rise in amplitude before deactivation. The initial increase in tail current amplitude may be due to fast recovery from inactivation, similar to that observed with HERG currents expressed in oocytes [688]
KV7.1 Single Channel Current
HODGKIN AND HUXLEY MODEL

MARKOV MODEL Kv7.1
HEART
KCNQ1 has a high level of expression in the heart where it co-assembles with minK (KCNE1) channels to give a slow delayed rectifier type current, IK,s. (Barhanin et al. 1996 [1074]; Sanguinetti et al. 1996 [1075]; Yang et al. 1997 [688]).
UTERUS
KCNQ expression has also been shown in the mouse and human uterus where KCNQ1 expression predominates throughout the oestrous cycle. KCNQ expression has also been found to change during pregnancy with the majority of KCNQ isoform expression decreases initially in early pregnancy before returning to robust levels at late pregnancy. Kv7 activators (flupirtine and retigabine) relaxed the human and mouse uterus ex vivo, but were more effective on the uteri of late pregnant mice and humans.
OTHER TISSUE
Kv7.1 is expressed in the stomach, small and large intestine, kidney and pancreas [722]
Smooth Muscle
Although Kv7.1 has been readily shown to be expressed in rodent and human vascular smooth muscle, the functional impact of this channel has remained enigmatic [1675]
DISTRIBUTION OF KV7.1 in NEURON
Staining of Kv7.1 in stratum lucidum could be attributable to granule cell mossy fiber axons or CA3 pyramidal cell apical dendrites.
KCNQ1 antibody also labeled most somata in the neocortex and thalamus, as well as glia-like processes in subcortical white matter tracts and the dentate granule cell layer [1682]
CARDIAC REPLOARIZATION
The role that IKs (the current which is mediated partly by KCNQ1) plays in cardiac repolarization is particularly important for its ability to counterbalance the depolarizing effect of enhanced L-type Ca2+ current during sympathetic stimulation of the heart (Bosch [1078], Burashnikov [1079], Farges [1080], Jost [1081]). This is highlighted by the finding that LQT1 mutations are usually symptomatically silent in many carriers until sudden exertion or emotional upset triggers cardiac events (Chen [1082], Zagotta [1083]). The properties of IKs constitute an important cardiac repolarization reserve invoked especially during elevated sympathetic tone (Nakashima [1086]).
BRAIN
They were first identified in the brain, where they modulate neuronal excitation [1677]
Arrythmia in Brain and Heart
That long QT syndrome mutations in KCNQ1 cause epilepsy reveals the dual arrhythmogenic potential of an ion channelopathy coexpressed in heart and brain and motivates a search for genetic diagnostic strategies to improve risk prediction and prevention of early mortality in persons with seizure disorders of unknown origin [1682]
Lange-Nielsen syndrome
Recent genetic analyses have revealed that most of the Jervell and Lange-Nielsen syndrome patients carried mutations in KCNQ1 that encodes the α-subunit of a voltage-gated cardiac potassium channel, KvLQT1 (a.k.a. Kv1.7). [682]
Romano Ward Syndrome
Romano Ward syndrome can be caused by mutations in genes for potassium channels, including KvLQT1 and HERG, sodium channels, a calcium channel, and other molecules associated with cardiac ion channels [683], [684], [685], [686], [687].
INSULIN
Kv7.1 is also present in pancreatic β-cells, where it is thought to be implicated in the regulation of insulin secretion [1671].
SALT& WATER TRANSPORT
In addition, Kv7.1 is expressed in several epithelia, where it is involved in salt and water transport [1672] Most importantly, the channel regulates gastric acid secretion and contributes to the release of potassium to the endolymph in the inner ear [1671]
KNOCKOUT MICE
Kv7.1 knock-out mice show gastric hyperplasia and are completely deaf [1673]
MUTATION
Mutations in the KCNQ1 gene are furthermore associated with long QT (LQT)4 syndrome, an inherited form of cardiac arrhythmia that can lead to cardiac arrest. In its recessive form, the Jervell and Lange-Nielsen syndrome, the disease additionally leads to hearing loss due to disturbances in the flow of potassium in the inner ear. The mechanism underlying the LQT syndrome is reflected in a loss of Kv7.1 function, frequently originating from trafficking disorders, and hence a decrease in number of channels in the plasma membrane [1670]
Airway Diseases (Asthma)
In airway smooth muscle, expression of KCNQ1, KCNQ4 and KCNQ5 appears to predominate in humans; however, KCNQ2 predominates in guinea pig with KCNQ1 being undetectable. This study found that both human and guinea pig airways were modulated by application of Kv7 activators and blockers, suggesting that Kv7 channels can regulate airway diameter and are likely to be responsible for maintaining the resting tone in the airways. Moreover, this study suggests that Kv7 enhancers may be useful bronchodilators in the treatment of airway diseases such as asthma [1677]
Gastric Juices
The cardiac K+ channel KCNQ1 is essential for gastric acid secretion [1752]
Ca2+/calmodulin (CaM) and PIP2
M-type channels can be variously composed of KCNQ1-5 subunits, and M current is known to be regulated by Ca2+/calmodulin (CaM) and PIP2. [249]
Antiarrythmic agent (clofilium)
Clofilium, a class III antiarrhythmic agent with the propensity to induce torsades de pointes, substantially inhibits the current [688]
KCNQ SUBUNITS
Stimulation of M1 receptors by 10 micro mol oxotremorine-M (Oxo-M) strongly reduced (to 0—10%) currents produced by KCNQ1—4 subunits expressed individually and also those produced by KCNQ2+KCNQ3 and KCNQ1+KCNE1 heteromers, which are thought to generate neuronal M currents (IK,M) and cardiac slow delayed rectifier currents (IK,s), respectively. (Selyanko [70])
Beta-adrenergic stimulation
IKs is distinguished from the rapidly activating delayed rectifier K+ current (IKr) by its gating kinetics, pharmacological sensitivity, and other properties, notably its considerable enhancement by beta-adrenergic stimulation (Han [1084], Sanguinetti [1085]).
Kv7.1 activators/blockers effect blood vessels
In several smooth muscle studies, Kv7.1-specific blockers such as chromanol 293B, HMR1556, L-768 673 and JNJ39490282 have had no contractile effect. However, recently Kv7.1 activators, such as R-L3 (L-364373) and mefenamic acid, relax precontracted rat blood vessels, which is abolished by application of Kv7.1-specific blockers. These findings suggest that Kv7.1 channels are functional in vascular smooth muscle but, because the Kv7.1-specific blockers have no effect, do not appear to contribute to resting vascular tone [1679]
INSULIN
insulin, a known activator of PI3K, also antagonizes Nedd4-2-mediated reductions in Kv7.1 currents [1670]
Linopirdine and XE991
linopirdine and XE991, produce membrane depolarization, and concomitant vasoconstriction by enhancing calcium influx through voltage-dependent calcium channels [1092]
KCNNE1
The potassium channel Kv7.1 (KCNQ1, KvLQT) plays an important role in a number of tissues where it associates with the auxiliary KCNE β-subunits. In the heart, Kv7.1, together with KCNE1, forms the delayed rectifier potassium current IKs, which is an important contributor to the repolarization of the cardiac action potential [689]
Whereas KCNQ1, which belongs to the Kv family of voltage-gated K+ channels, reconstitutes a rapidly activating voltage-dependent K+ conductance, cotransfection of the KCNE1 regulatory subunit confers the characteristic delayed slowly activating gating kinetics as well as the cAMP/protein kinase A (PKA) pathway-dependent modulation of the native current, IKs (Barhani [10], Kurokawa [1076], Marx [1077], Sanguinetti [689]).
KCNE3
In epithelia, Kv7.1 associates with the KCNE3 expression product to generate a constitutively active, voltage-independent channel crucial for fluid secretion/accumulation [1675]
293B
Retigabine
Additionally, Leu-272 in S5, Leu-314 within the inner pore loop, and Leu-338 in S6 of the neighbouring subunit are of importance for the binding of retigabine. These four amino acids are not found in Kv7.1, thus explaining why this subtype is insensitive to retigabine-induced enhancement [1675]
E-4031, 4-aminopurine, tetraethylammonium, and clofilium

MinK
Coexpression of Kv1.7 with MinK induces the cardiac current IKs. [689]
Nedd4-2

References
Selyanko AA
et al.
Inhibition of KCNQ1-4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors.
J. Physiol. (Lond.),
2000
Feb
1
, 522 Pt 3 (349-55).
Imredy JP
et al.
Modeling of the adrenergic response of the human IKs current (hKCNQ1/hKCNE1) stably expressed in HEK-293 cells.
Am. J. Physiol. Heart Circ. Physiol.,
2008
Nov
, 295 (H1867-81).
Seebohm G
et al.
Mutation of colocalized residues of the pore helix and transmembrane segments S5 and S6 disrupt deactivation and modify inactivation of KCNQ1 K+ channels.
J. Physiol. (Lond.),
2005
Mar
1
, 563 (359-68).
Boulet IR
et al.
Role of the S6 C-terminus in KCNQ1 channel gating.
J. Physiol. (Lond.),
2007
Dec
1
, 585 (325-37).
Tinel N
et al.
KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel.
EMBO J.,
2000
Dec
1
, 19 (6326-30).
Seebohm G
et al.
A kinetic study on the stereospecific inhibition of KCNQ1 and I(Ks) by the chromanol 293B.
Br. J. Pharmacol.,
2001
Dec
, 134 (1647-54).
Sato A
et al.
Novel mechanisms of trafficking defect caused by KCNQ1 mutations found in long QT syndrome.
J. Biol. Chem.,
2009
Dec
11
, 284 (35122-33).
Bal M
et al.
Ca2+/calmodulin disrupts AKAP79/150 interactions with KCNQ (M-Type) K+ channels.
J. Neurosci.,
2010
Feb
10
, 30 (2311-23).
Peroz D
et al.
Kv7.1 (KCNQ1) properties and channelopathies.
J. Physiol. (Lond.),
2008
Apr
1
, 586 (1785-9).
Jentsch TJ
Neuronal KCNQ potassium channels: physiology and role in disease.
Nat. Rev. Neurosci.,
2000
Oct
, 1 (21-30).
Neyroud N
et al.
A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome.
Nat. Genet.,
1997
Feb
, 15 (186-9).
Lehnart SE
et al.
Inherited arrhythmias: a National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affe
Circulation,
2007
Nov
13
, 116 (2325-45).
Wang Q
et al.
Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias.
Nat. Genet.,
1996
Jan
, 12 (17-23).
Curran ME
et al.
A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome.
Cell,
1995
Mar
10
, 80 (795-803).
Napolitano C
et al.
Genetic testing in the long QT syndrome: development and validation of an efficient approach to genotyping in clinical practice.
JAMA,
2005
Dec
21
, 294 (2975-80).
Yang WP
et al.
KvLQT1, a voltage-gated potassium channel responsible for human cardiac arrhythmias.
Proc. Natl. Acad. Sci. U.S.A.,
1997
Apr
15
, 94 (4017-21).
Sanguinetti MC
et al.
Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel.
Nature,
1996
Nov
7
, 384 (80-3).
Robbins J
KCNQ potassium channels: physiology, pathophysiology, and pharmacology.
Pharmacol. Ther.,
2001
Apr
, 90 (1-19).
Barhanin J
et al.
K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current.
Nature,
1996
Nov
7
, 384 (78-80).
Sanguinetti MC
Dysfunction of delayed rectifier potassium channels in an inherited cardiac arrhythmia.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (406-13).
Kurokawa J
et al.
Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel.
Proc. Natl. Acad. Sci. U.S.A.,
2003
Feb
18
, 100 (2122-7).
Marx SO
et al.
Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel.
Science,
2002
Jan
18
, 295 (496-9).
Bosch RF
et al.
beta3-Adrenergic regulation of an ion channel in the heart-inhibition of the slow delayed rectifier potassium current I(Ks) in guinea pig ventricular myocytes.
Cardiovasc. Res.,
2002
Dec
, 56 (393-403).
Burashnikov A
et al.
Block of I(Ks) does not induce early afterdepolarization activity but promotes beta-adrenergic agonist-induced delayed afterdepolarization activity.
J. Cardiovasc. Electrophysiol.,
2000
Apr
, 11 (458-65).
Farges JP
et al.
Relationship between atrial and ventricular rates of fibrillation and cardiac contractile tissue effective refractory periods in the dog.
Br. J. Pharmacol.,
1978
Aug
, 63 (587-91).
Jost N
et al.
Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle.
Circulation,
2005
Sep
6
, 112 (1392-9).
Chen S
et al.
KCNQ1 mutations in patients with a family history of lethal cardiac arrhythmias and sudden death.
Clin. Genet.,
2003
Apr
, 63 (273-82).
Zagotta WN
et al.
Shaker potassium channel gating. III: Evaluation of kinetic models for activation.
J. Gen. Physiol.,
1994
Feb
, 103 (321-62).
Han W
et al.
Slow delayed rectifier current and repolarization in canine cardiac Purkinje cells.
Am. J. Physiol. Heart Circ. Physiol.,
2001
Mar
, 280 (H1075-80).
Sanguinetti MC
et al.
Isoproterenol antagonizes prolongation of refractory period by the class III antiarrhythmic agent E-4031 in guinea pig myocytes. Mechanism of action.
Circ. Res.,
1991
Jan
, 68 (77-84).
Nakashima H
et al.
In vivo electrophysiological effects of a selective slow delayed-rectifier potassium channel blocker in anesthetized dogs: potential insights into class III actions.
Cardiovasc. Res.,
2004
Mar
1
, 61 (705-14).
Ohya S
et al.
Molecular variants of KCNQ channels expressed in murine portal vein myocytes: a role in delayed rectifier current.
Circ. Res.,
2003
May
16
, 92 (1016-23).
Andersen MN
et al.
A Phosphoinositide 3-Kinase (PI3K)-serum- and glucocorticoid-inducible Kinase 1 (SGK1) Pathway Promotes Kv7.1 Channel Surface Expression by Inhibiting Nedd4-2 Protein.
J. Biol. Chem.,
2013
Dec
27
, 288 (36841-54).
Yamagata K
et al.
Voltage-gated K+ channel KCNQ1 regulates insulin secretion in MIN6 β-cell line.
Biochem. Biophys. Res. Commun.,
2011
Apr
15
, 407 (620-5).
Dedek K
et al.
Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract.
Pflugers Arch.,
2001
Sep
, 442 (896-902).
Lee MP
et al.
Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice.
J. Clin. Invest.,
2000
Dec
, 106 (1447-55).
Ullrich S
et al.
Effects of I(Ks) channel inhibitors in insulin-secreting INS-1 cells.
Pflugers Arch.,
2005
Dec
, 451 (428-36).
Jepps TA
et al.
One man's side effect is another man's therapeutic opportunity: targeting Kv7 channels in smooth muscle disorders.
Br. J. Pharmacol.,
2013
Jan
, 168 (19-27).
Silva JR
et al.
A multiscale model linking ion-channel molecular dynamics and electrostatics to the cardiac action potential.
Proc. Natl. Acad. Sci. U.S.A.,
2009
Jul
7
, 106 (11102-6).
Brueggemann LI
et al.
Kv7 potassium channels in airway smooth muscle cells: signal transduction intermediates and pharmacological targets for bronchodilator therapy.
Am. J. Physiol. Lung Cell Mol. Physiol.,
2012
Jan
, 302 (L120-32).
McCallum LA
et al.
The contribution of Kv7 channels to pregnant mouse and human myometrial contractility.
J. Cell. Mol. Med.,
2011
Mar
, 15 (577-86).
Chadha PS
et al.
Pharmacological dissection of K(v) 7.1 channels in systemic and pulmonary arteries.
,
2012
Jan
17
, ().
Goldman AM
et al.
Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death.
Sci Transl Med,
2009
Oct
14
, 1 (2ra6).
Loussouarn G
et al.
Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels.
EMBO J.,
2003
Oct
15
, 22 (5412-21).
Smith JA
et al.
Structural models for the KCNQ1 voltage-gated potassium channel.
Biochemistry,
2007
Dec
11
, 46 (14141-52).
Grahammer F
et al.
The cardiac K+ channel KCNQ1 is essential for gastric acid secretion.
Gastroenterology,
2001
May
, 120 (1363-71).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/23/ , accessed on 2026 Feb 23