K
Description: Potassium channel
Potassium channels represent the most complex class of voltage-gated ion channels from both functional and structural standpoints. Four sequence-related potassium channel genes - shaker, shaw, shab, and shal - have been identified in Drosophila, and each has been shown to have human homolog(s). For example Kv1 (homologous to Drosophila Shaker), Kv2 (Shab), Kv3 (Shaw), Kv4 (Shal), Kv5, Kv6, , Kv8 and the other Kv channels listed in Channelpedia.
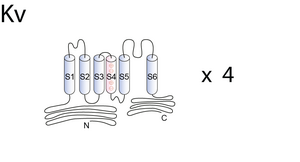
Outward rectifiers constitute a large class of voltage-dependent K+ channels. They have six transmembrane domains (S1–S6), one very positively charged (S4), and a typical pore region situated between S5 and S6 [737],[738], [739], [740]. Sequence similarities between members of the Kv family were initially used to define the different subfamilies of alpha subunits. The different members within a given subfamily share only a percentage of 30 –50% with members of others subfamilies. To date 20 functional voltage-gated potassium channels alpha subunits have been described. They belong to six subfamilies designated Kv1 (Shaker), Kv2 (Shab), Kv3 (Shaw), Kv4 (Shal), KvLQT, and EAG. The diversity of potassium channel functions comes from the diversity of potassium channel genes and is increased by alternate splicing (10, 11), regulatory beta subunits (12–14) and heteromultimerization between the different alpha subunits of the same subfamily [741], [733], [734], or sometimes between different subfamilies [742], [743].
Visual Representation of Kv Structure
Methodology for visual representation of structure available here
The following is paraphrased from [664]: Kv channels are composed of four subunits that surround the central ion permeation pathway. Each subunit has six transmembrane domains (S1–S6) and a pore region containing the signature sequence GYG characteristic for potassium channels [667], [619]. Post-translational assembly of tetrameric Kv channels takes place in the ER2 membrane; sub- sequently the channels traffic to the plasma membrane [619], [668]. A highly conserved sequence in the cytoplasmic N terminus of Kv channels, the tetramerization domain or T1 domain, has been shown to play an important role in channel assembly [619], [669]. The T1 domain contains some of the molecular determinants for subfamily-specific homo- or heterotetrameric assembly of Kv alpha-subunits [669], [660],[598], [670]. The most striking difference between the T1 domains of Kv1 (Shaker) and Kv2–4 (non-Shaker) channels is the presence of intersubunit-coordinated Zn2+ ions at the assembly interface in non-Shaker channels. The Zn2+ ions are coordinated by a C3H1 motif embedded in a conserved sequence motif (HX5CX20CC) of the T1 domain, which is located near the distal end of the N terminus [671], [672], [673]. These four amino acids are exposed on the subunit interface, with one histidine and two cysteine residues belonging to one subunit and one cysteine residue belonging to the neighboring subunit [671]. The T1 domain facilitates tetrameric assembly of Kv channels. Kv subunits in which the T1 has been deleted have been reported to assemble in a promiscuous way via their transmembrane domains and to form stable, functional channels, but both the rates and the efficiency of channel assembly are significantly lower in the mutant channels as compared with their wild-type counterparts [668], [674]. Heteromeric assembly of channel subunits is a potential source of diversity of K+ channel properties.
Eight different voltage-gated K+ (Kv)3 Shaker-related channel subfamilies (Kv1–Kv6 and Kv8–Kv9) have been identified based on the degree of sequence homology [606]. Fully assembled Kv channels are composed of four α-subunits arranged around a central pore. Each α-subunit consists of six transmembrane segments S1–S6 with a cytoplasmic N and C terminus. The N terminus contains the T1 domain, a tetramerization domain that facilitates the assembly of α-subunits into functional channels. The presence of a T1 domain is not absolutely required for channel assembly because subunits without a T1 domain could also assemble into a functional tetramer, although less efficiently [677], [678], [679]. However, the T1 domain not only promotes but also restricts the formation of possible homo- and heterotetramers by preventing incompatible subunits from assembling [680], [660]. When four compatible T1 domains assemble, they are arranged with the same 4-fold symmetry as the transmembrane segments, forming a hanging gondola structure [681].
Biophysics
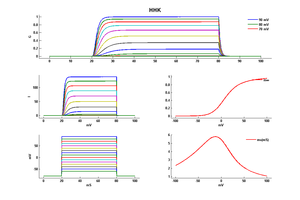
Model HHK (ID=1)
| Animal | Squid | |
| CellType | giant Axon | |
| Age | 0 Days | |
| Temperature | 9.3°C | |
| Reversal | -78.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [262] A L Hodgkin et. al; Bull. Math. Biol. 1990 | |
| mpower | 4.0 | |
| m Alpha | (0.01*(10-v))/(exp((10-v)/10) - 1.0) If v neq 10 | |
| m Beta | 0.125 * (exp(-v/80)) | |
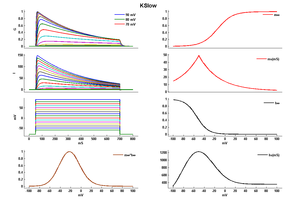
Model KSlow (ID=30)
This is a better model because I think so
| Animal | rat | |
| CellType | Neocortical L5PC | |
| Age | 15 Days | |
| Temperature | 23.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [264] A Korngreen et. al; J. Physiol. (Lond.) 2000 Jun 15 | |
| mpower | 2.0 | |
| m Inf | (1/(1 + exp(-(v+14)/14.6))) | |
| m Tau | (1.25+175.03*exp(-v * -0.026)) If v lt -50 | |
| m Tau | (1.25+13*exp(-v*0.026)) If v gteq -50 | |
| hpower | 1.0 | |
| h Inf | 1/(1 + exp(-(v+54)/-11)) | |
| h Tau | 360+(1010+24*(v+55))*exp(-((v+75)/48)^2) | |
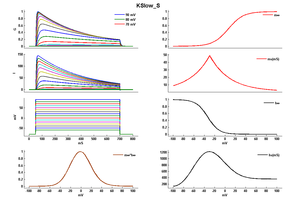
Model KSlow_S (ID=31)
Modified KSlow model (V shift = -21)
| Animal | rat | |
| CellType | Neocortical L5PC | |
| Age | 15 Days | |
| Temperature | 23.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [264] A Korngreen et. al; J. Physiol. (Lond.) 2000 Jun 15 | |
| mpower | 2.0 | |
| m Inf | (1/(1 + exp(-((v-21)+14)/14.6))) | |
| m Tau | (1.25+175.03*exp(-(v-21) * -0.026)) If v lt -29 | |
| m Tau | (1.25+13*exp(-(v-21)*0.026)) If v gteq -29 | |
| hpower | 1.0 | |
| h Inf | 1/(1 + exp(-((v-21)+54)/-11)) | |
| h Tau | 360+(1010+24*((v-21)+55))*exp(-(((v-21)+75)/48)^2) | |
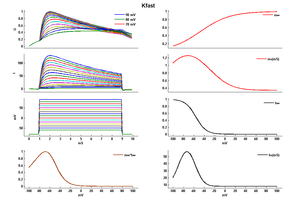
Model Kfast (ID=32)
| Animal | rat | |
| CellType | Neocortical L5PC | |
| Age | 15 Days | |
| Temperature | 23.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [264] A Korngreen et. al; J. Physiol. (Lond.) 2000 Jun 15 | |
| mpower | 1.0 | |
| m Inf | 1/(1 + exp(-(v+47)/29)) | |
| m Tau | (0.34+0.92*exp(-((v+71)/59)^2)) | |
| hpower | 1.0 | |
| h Inf | 1/(1 + exp(-(v+56)/-10)) | |
| h Tau | (8+49*exp(-((v+73)/23)^2)) | |
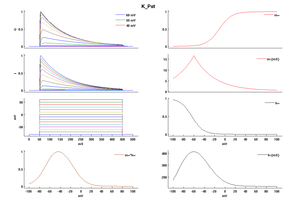
Model K_Pst (ID=48)
| Animal | rat | |
| CellType | Neocortical L5PC | |
| Age | 15 Days | |
| Temperature | 23.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [264] A Korngreen et. al; J. Physiol. (Lond.) 2000 Jun 15 | |
| mpower | 2.0 | |
| m Inf | (1/(1 + exp(-(v+1)/12))) | |
| m Tau | (1.25+175.03*exp(-v * -0.026))/qt If v lt -50 | |
| m Tau | ((1.25+13*exp(-v*0.026)))/qt If v gteq -50 | |
| hpower | 1.0 | |
| h Inf | 1/(1 + exp(-(v+54)/-11)) | |
| h Tau | (360+(1010+24*(v+55))*exp(-((v+75)/48)^2))/qt | |
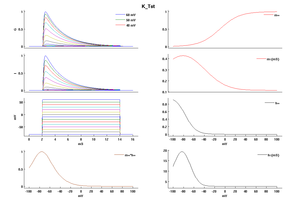
Model K_Tst (ID=49)
| Animal | rat | |
| CellType | Neocortical L5PC | |
| Age | 15 Days | |
| Temperature | 23.0°C | |
| Reversal | -85.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [264] A Korngreen et. al; J. Physiol. (Lond.) 2000 Jun 15 | |
| mpower | 4.0 | |
| m Inf | 1/(1 + exp(-(v+0)/19)) | |
| m Tau | (0.34+0.92*exp(-((v+71)/59)^2))/qt | |
| hpower | 1.0 | |
| h Inf | 1/(1 + exp(-(v+66)/-10)) | |
| h Tau | (8+49*exp(-((v+73)/23)^2))/qt | |
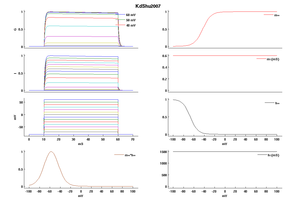
Model KdShu2007 (ID=50)
| Animal | rat | |
| CellType | L5PC | |
| Age | 17 Days | |
| Temperature | 23.0°C | |
| Reversal | -85.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [1499] Yousheng Shu et. al; Proc. Natl. Acad. Sci. U.S.A. 2007 Jul 3 | |
| mpower | 1.0 | |
| m Inf | 1-1/(1+exp((v- -43)/8)) | |
| m Tau | 0.6 | |
| hpower | 1.0 | |
| h Inf | 1/(1+exp((v- -67)/7.3)) | |
| h Tau | 1500 | |
Voltage-gated potassium channels of the Kv family are strongly expressed in the mammalian central nervous system, in the immune system, in muscle cells and in many other cell types. Most neurons express multiple Kv channel subtypes belonging to one or more subfamilies [496], [638], [665].
Their diverse functions include regulating neurotransmitter release, heart rate, insulin secretion, neuronal excitability, epithelial electrolyte transport, smooth muscle contraction, and cell volume.
Voltage gated potassium channels play a key role in controlling neuronal excitability and regulate a variety of electrophysiological properties, such as the interspike membrane potential, the waveform of the action potential and the firing frequency [666].
Heterologous expression of homotetrameric channels of the Kv1-Kv4 subfamilies display distinctive gating characteristics associated with variations in the primary sequence. In addition, gating is known to be modified through assembly of heterotetramers consisting either of different alpha-subunits [649] or alpha-subunits with accessory beta-subunits [312].
References
Hodgkin AL
et al.
A quantitative description of membrane current and its application to conduction and excitation in nerve. 1952.
Bull. Math. Biol.,
1990
, 52 (25-71; discussion 5-23).
Korngreen A
et al.
Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients.
J. Physiol. (Lond.),
2000
Jun
15
, 525 Pt 3 (621-39).
Nusser Z
Variability in the subcellular distribution of ion channels increases neuronal diversity.
Trends Neurosci.,
2009
May
, 32 (267-74).
Cao XH
et al.
Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor.
J. Neurochem.,
2010
Sep
1
, 114 (1460-75).
Park WS
et al.
Patho-, physiological roles of voltage-dependent K+ channels in pulmonary arterial smooth muscle cells.
J Smooth Muscle Res,
2010
, 46 (89-105).
Coetzee WA
et al.
Molecular diversity of K+ channels.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (233-85).
Li M
et al.
Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel.
Science,
1992
Aug
28
, 257 (1225-30).
Gutman GA
et al.
International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels.
Pharmacol. Rev.,
2005
Dec
, 57 (473-508).
Lu J
et al.
T1-T1 interactions occur in ER membranes while nascent Kv peptides are still attached to ribosomes.
Biochemistry,
2001
Sep
18
, 40 (10934-46).
Jan LY
et al.
Voltage-gated and inwardly rectifying potassium channels.
J. Physiol. (Lond.),
1997
Dec
1
, 505 ( Pt 2) (267-82).
Xu J
et al.
Assembly of voltage-gated potassium channels. Conserved hydrophilic motifs determine subfamily-specific interactions between the alpha-subunits.
J. Biol. Chem.,
1995
Oct
20
, 270 (24761-8).
Mederos Y Schnitzler M
et al.
Mutation of histidine 105 in the T1 domain of the potassium channel Kv2.1 disrupts heteromerization with Kv6.3 and Kv6.4.
J. Biol. Chem.,
2009
Feb
13
, 284 (4695-704).
Hille B
Ionic selectivity of Na and K channels of nerve membranes.
Membranes,
1975
, 3 (255-323).
Barry DM
et al.
Myocardial potassium channels: electrophysiological and molecular diversity.
Annu. Rev. Physiol.,
1996
, 58 (363-94).
Robinson JM
et al.
Coupled tertiary folding and oligomerization of the T1 domain of Kv channels.
Neuron,
2005
Jan
20
, 45 (223-32).
Bixby KA
et al.
Zn2+-binding and molecular determinants of tetramerization in voltage-gated K+ channels.
Nat. Struct. Biol.,
1999
Jan
, 6 (38-43).
Jahng AW
et al.
Zinc mediates assembly of the T1 domain of the voltage-gated K channel 4.2.
J. Biol. Chem.,
2002
Dec
6
, 277 (47885-90).
Strang C
et al.
The role of Zn2+ in Shal voltage-gated potassium channel formation.
J. Biol. Chem.,
2003
Aug
15
, 278 (31361-71).
Tu L
et al.
Voltage-gated K+ channels contain multiple intersubunit association sites.
J. Biol. Chem.,
1996
Aug
2
, 271 (18904-11).
Bocksteins E
et al.
Conserved negative charges in the N-terminal tetramerization domain mediate efficient assembly of Kv2.1 and Kv2.1/Kv6.4 channels.
J. Biol. Chem.,
2009
Nov
13
, 284 (31625-34).
Kobertz WR
et al.
K+ channels lacking the 'tetramerization' domain: implications for pore structure.
Nat. Struct. Biol.,
1999
Dec
, 6 (1122-5).
Zerangue N
et al.
An artificial tetramerization domain restores efficient assembly of functional Shaker channels lacking T1.
Proc. Natl. Acad. Sci. U.S.A.,
2000
Mar
28
, 97 (3591-5).
Lee TE
et al.
Structural determinant for assembly of mammalian K+ channels.
Biophys. J.,
1994
Mar
, 66 (667-73).
Kobertz WR
et al.
Hanging gondola structure of the T1 domain in a voltage-gated K(+) channel.
Biochemistry,
2000
Aug
29
, 39 (10347-52).
Sheng M
et al.
Presynaptic A-current based on heteromultimeric K+ channels detected in vivo.
Nature,
1993
Sep
2
, 365 (72-5).
Wang H
et al.
Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons.
Nature,
1993
Sep
2
, 365 (75-9).
Pongs O
Structure-function studies on the pore of potassium channels.
J. Membr. Biol.,
1993
Oct
, 136 (1-8).
Heginbotham L
et al.
Mutations in the K+ channel signature sequence.
Biophys. J.,
1994
Apr
, 66 (1061-7).
MacKinnon R
Pore loops: an emerging theme in ion channel structure.
Neuron,
1995
May
, 14 (889-92).
Pascual JM
et al.
K+ pore structure revealed by reporter cysteines at inner and outer surfaces.
Neuron,
1995
May
, 14 (1055-63).
Christie MJ
et al.
Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits.
Neuron,
1990
Mar
, 4 (405-11).
Shahidullah M
et al.
Slow inactivation conserved in heteromultimeric voltage-dependent K+ channels between Shaker (Kv1) and Shaw (Kv3) subfamilies.
FEBS Lett.,
1995
Sep
11
, 371 (307-10).
Chen ML
et al.
Heteromultimeric interactions among K+ channel subunits from Shaker and eag families in Xenopus oocytes.
Neuron,
1996
Sep
, 17 (535-42).
Shu Y
et al.
Selective control of cortical axonal spikes by a slowly inactivating K+ current.
Proc. Natl. Acad. Sci. U.S.A.,
2007
Jul
3
, 104 (11453-8).
Contributors: Rajnish Ranjan, Michael Schartner
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/188/ , accessed on 2026 Mar 07