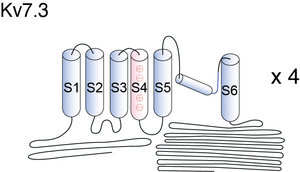
Kv7.3
Description: potassium voltage-gated channel, KQT-like subfamily, member 3 Gene: Kcnq3 Alias: KV7.3, EBN2, BFNC2, KCNQ3
KV7.3, encoded by KCNQ3, is a members of The potassium voltage-gated channel KQT-like subfamily. Kv7.3 is also known as: CNQ3; EBN2; BFNC2; FLJ37386; FLJ38392; DKFZp686C0248. Kv7.3 assembles with Kv7.2 to form the M channel, a slowly activating and deactivating potassium channel that plays a critical role in the regulation of neuronal excitability. NCBI [690].
Experimental data
Rat Kv7.3 gene in CHO host cells datasheet |
||
|
Click for details 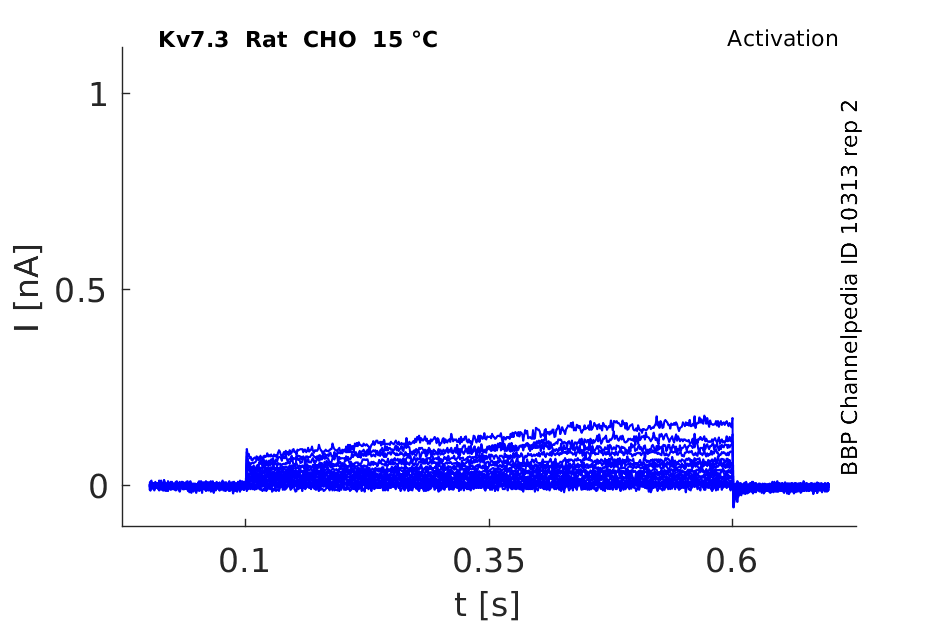
15 °Cshow 79 cells |
Click for details 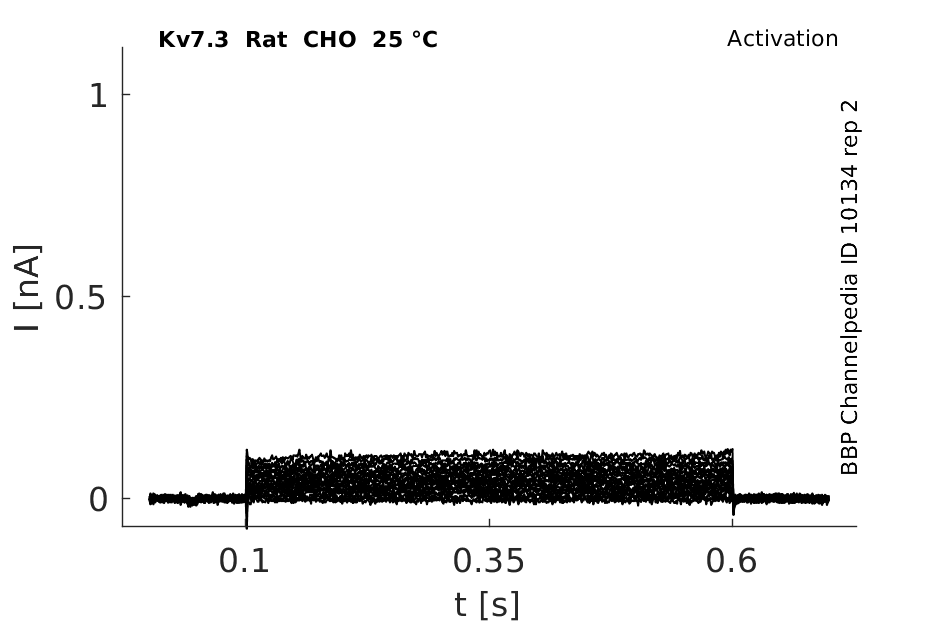
25 °Cshow 63 cells |
Click for details 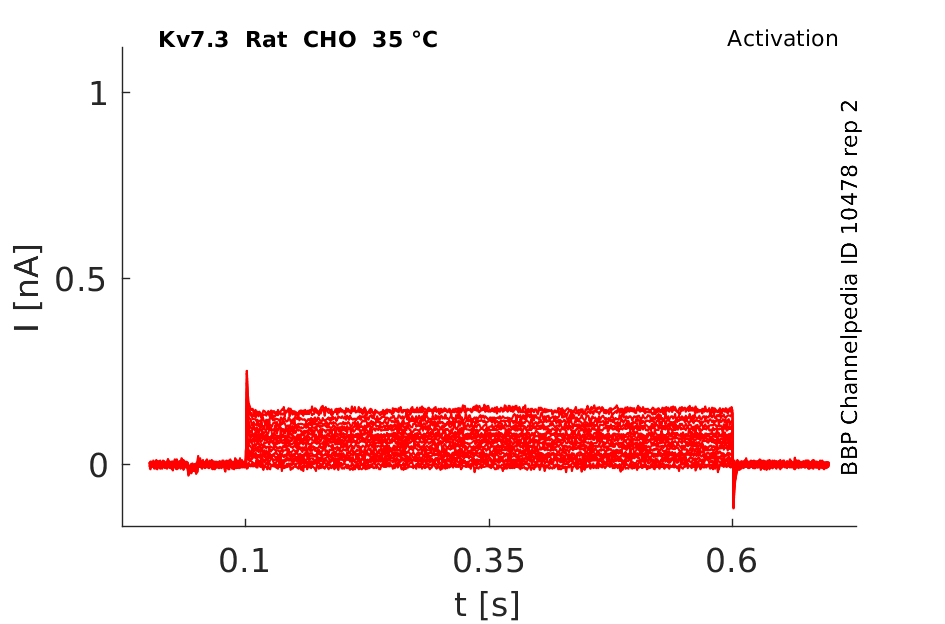
35 °Cshow 86 cells |
From an evolutionary perspective, KCNQ2 and KCNQ3 were the last KCNQ subunits to emerge, coincident with the apparition of myelin [701].
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_004519.4 | 11583 | |
| Mouse | NM_152923.3 | 11824 | |
| Rat | NM_031597.4 | 5798 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv7.3 Structure
Methodology for visual representation of structure available here
Basic structure of KCNQ2/3 proteins can be seen in figure 1 of [464] KCNQ3 is unusual because it has an alanine in the inner vestibule, three residues upstream of the signature GYG (see Fig.1A in [698]).
It is generally thought that KCNQ2/3 surface expression is governed by the intracellularC-terminal region, and that the pore region does not have any significant role in trafficking [705], [707], [708], [709], [73].
Kv7.3 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
KCNQ2/KCNQ3 (See also Kv7.2 for more info)
KCNQ2/KCNQ3 heteromers yield currents with the properties of the M-current, see figure 2 in [464].
Mutations in either KCNQ2 or KCNQ3 can cause the same phenotype (BFNC), and the expression of both subunits overlaps extensively, so they may combine to form a single channel. Expression of both subunits leads to larger currents with slightly changed gating kinetics and sensitivity to inhibitors. Furthermore, a KCNQ3 mutant modelled on a dominant-negative KCNQ1 mutation found in RWS inhibited KCNQ2 currents in a DOMINANT-NEGATIVE fashion [704]
SINGLE CHANNEL CONDUCTANCE
Single-channel and noise analysis indicated that homomeric KCNQ2 and KCNQ3 channels have conductances of roughly 18 and 7 pS, respectively. Co-expression did not increase the single-channel conductance, which varied between 8 and 22 ps. This variation suggested the formation of heteromers with different stoichiometries [463]
KCNQ2/3 in CHO cells

KCNQ2 and KCNQ3 are coexpressed on the cell bodies and dendrites of many hippocampal and cortical neurons. [461]
DISTRIBUTION OF Kv7.3 CHANNEL IN NEURON
In the hippocampal formation and cerebral cortex, KCNQ2 and KCNQ3 staining was detected on the somata and dendrites of many polymorphic and pyramidal neurons. Confocal immunofluorescence microscopy revealed that this somatodendritic staining was punctate in character and appeared to label both the cell surface and intracellular components (C). In the hippocampal formation, hilar polymorphic cells. ( E and H), CA3 pyramidal cells (F), and subicular pyramidal cells (G) exhibited somatodendritic staining for both subunits.
KCNQ2 and KCNQ3 proteins are colocalized in a somatodendritic pattern on pyramidal and polymorphic neurons in the human cortex and hippocampus. Immunoreactivity for KCNQ2, but not KCNQ3, is also prominent in some terminal fields, suggesting a presynaptic role for a distinct subgroup of M-channels in the regulation of action potential propagation and neurotransmitter release [1681]
The KCNQ2/3 subunits acquired an ankyrin G-binding motif, that allows them to concentrate at the nodes of Ranvier and the axonal initial segment (AIS) [702], [703]. KCNQ3 is a major determinant of M-channels location to the AIS [703] and displays a predominant intracellular distribution, whereas its combination with KCNQ2 leads to a large increase in axonal surface expression [706].
Spontaneous mutations in Kv7.3, some producing a mere 25% reduction in function, cause human neonatal epilepsy [704], [705].
KCNQ2 and KCNQ3 play an important role in neonatal epilepsy. [462]
BNFC
Mutations causing benign familial neonatal convulsions were found to cause only slight reductions in current compared with wild-type controls, suggesting that small differences in the activity of these KCNQ channels in vivo might be sufficient to cause epilepsy [704]
M channel currents are inhibited by M1 muscarinic acetylcholine receptors and activated by retigabine, a anti-convulsant drug. NCBI
Expression of KCNQ2 and KCNQ3 individually yields only small currents, whereas their coexpression yields heteromeric currents 10-fold larger [695].
Extracellular H+ ions
Whole-cell and single-channel recordings demonstrated that extracellular H+ ions effect heterologously expressed KCNQ2/3 channels in the following way: KCNQ2/3 current was inhibited by H+ ions with an IC50 of 52 nM (pH 7.3) at -60 mV, rising to 2 microM (pH 5.7) at -10 mV. Neuronal M-current exhibited a similar sensitivity. I.e. extracellular H+ ions affected two distinct properties of KCNQ2/3 current: the maximum current attainable upon depolarization (Imax) and the voltage dependence of steady-state activation. [66]
Mepyramine and Diphenhydramine
Mepyramine and diphenhydramine, two structurally related first-generation antihistamines, can act as potent KCNQ/M channel blockers. Extracellular application of these drugs quickly and reversibly reduced KCNQ2/Q3 currents heterologously expressed in HEK293 cells. [72]
Hydroxyl-Containing Residue at the 315 Position
Most of the wild-type KCNQ3 homomers, being well expressed at the plasma membrane, are functionally silent. Rearrangements of the pore-loop architecture induced by the presence of a hydroxyl-containing residue at the 315 position unlocks the channels into a conductive conformation. [73]
Meclofenamate and Diclofenac
Meclofenamic acid (meclofenamate) and diclofenac, two related molecules previously used as anti-inflammatory drugs, act as KCNQ2/Q3 channel openers. Extracellular application of meclofenamate (EC(50) = 25 microM) and diclofenac (EC(50) = 2.6 microM) resulted in the activation of KCNQ2/Q3 K(+) currents by causing a hyperpolarizing shift of the voltage activation curve and markedly slowing the deactivation kinetics. The effects of the drugs were stronger on KCNQ2 than on KCNQ3 channel alpha subunits but they did not enhance KCNQ1 K(+) currents. Both openers increased KCNQ2/Q3 current amplitude at physiologically relevant potentials and led to hyperpolarization of the resting membrane potential. [78]
KCNE1
KCNE1 slowed KCNQ2/3 currents and decreased their magnitude [1680]. However no significant effects have also been recorded when KCNE1 was co-expressed at levels that suffice to alter KCNQ1. An interaction of KCNQ2/3 with either KCNQ1 or KCNE1 would also seem physiologically irrelevant as neither has been detected in the CNS [704]
PIP
Native M-channels and expressed Kv7.2 + 7.3 channels are inhibited by stimulating Gq/11-coupled receptors – prototypically the M1 muscarinic acetylcholine receptor (M1-mAChR). The channels require membrane phosphatidylinositol-4,5-bisphosphate (PIP2) to open and the effects of mAChR stimulation result primarily from the reduction in membrane PIP2 levels following Gq/phospholipase C-catalysed PIP2 hydrolysis. However, in sympathetic neurons, M-current inhibition by bradykinin appears to be mediated through the release and action of intracellular Ca2+ by inositol-1,4,5-trisphosphate (IP3), a product of PIP2 hydrolysis, rather than by PIP2 depletion [1744]
References
Selective interaction of syntaxin 1A with KCNQ2: possible implications for specific modulation of presynaptic activity.
PLoS ONE, 2009 , 4 (e6586).
Xiong Q
et al.
Combinatorial augmentation of voltage-gated KCNQ potassium channels by chemical openers.
Proc. Natl. Acad. Sci. U.S.A.,
2008
Feb
26
, 105 (3128-33).
Schenzer A
et al.
Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine.
J. Neurosci.,
2005
May
18
, 25 (5051-60).
Prole DL
et al.
Mechanisms underlying modulation of neuronal KCNQ2/KCNQ3 potassium channels by extracellular protons.
J. Gen. Physiol.,
2003
Dec
, 122 (775-93).
Schwake M
et al.
A carboxy-terminal domain determines the subunit specificity of KCNQ K+ channel assembly.
EMBO Rep.,
2003
Jan
, 4 (76-81).
Gamper N
et al.
Subunit-specific modulation of KCNQ potassium channels by Src tyrosine kinase.
J. Neurosci.,
2003
Jan
1
, 23 (84-95).
Tatulian L
et al.
Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine.
J. Neurosci.,
2001
Aug
1
, 21 (5535-45).
Selyanko AA
et al.
Inhibition of KCNQ1-4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors.
J. Physiol. (Lond.),
2000
Feb
1
, 522 Pt 3 (349-55).
Liu B
et al.
Antihistamine mepyramine directly inhibits KCNQ/M channel and depolarizes rat superior cervical ganglion neurons.
Neuropharmacology,
2008
Mar
, 54 (629-39).
Zaika O
et al.
Determinants within the turret and pore-loop domains of KCNQ3 K+ channels governing functional activity.
Biophys. J.,
2008
Dec
, 95 (5121-37).
Peretz A
et al.
Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties.
Mol. Pharmacol.,
2005
Apr
, 67 (1053-66).
Dedek K
et al.
Myokymia and neonatal epilepsy caused by a mutation in the voltage sensor of the KCNQ2 K+ channel.
Proc. Natl. Acad. Sci. U.S.A.,
2001
Oct
9
, 98 (12272-7).
Jentsch TJ
Neuronal KCNQ potassium channels: physiology and role in disease.
Nat. Rev. Neurosci.,
2000
Oct
, 1 (21-30).
Brown DA
et al.
Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone.
Nature,
1980
Feb
14
, 283 (673-6).
Bailey SD
et al.
Variation at the NFATC2 locus increases the risk of thiazolidinedione-induced edema in the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) study.
Diabetes Care,
2010
Oct
, 33 (2250-3).
Talmud PJ
et al.
Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip.
Am. J. Hum. Genet.,
2009
Nov
, 85 (628-42).
Hahn A
et al.
Sodium and potassium channel dysfunctions in rare and common idiopathic epilepsy syndromes.
Brain Dev.,
2009
Aug
, 31 (515-20).
Wang HS
et al.
KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel.
Science,
1998
Dec
4
, 282 (1890-3).
Gómez-Posada JC
et al.
A pore residue of the KCNQ3 potassium M-channel subunit controls surface expression.
J. Neurosci.,
2010
Jul
7
, 30 (9316-23).
Rose JE
et al.
Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score.
Mol. Med.,
2010 Jul-Aug
, 16 (247-53).
Shah MM
et al.
Molecular correlates of the M-current in cultured rat hippocampal neurons.
J. Physiol. (Lond.),
2002
Oct
1
, 544 (29-37).
Hill AS
et al.
Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates.
PLoS Genet.,
2008
Dec
, 4 (e1000317).
Pan Z
et al.
A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon.
J. Neurosci.,
2006
Mar
8
, 26 (2599-613).
Rasmussen HB
et al.
Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment.
J. Cell. Sci.,
2007
Mar
15
, 120 (953-63).
Schroeder BC
et al.
Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy.
Nature,
1998
Dec
17
, 396 (687-90).
Maljevic S
et al.
C-terminal interaction of KCNQ2 and KCNQ3 K+ channels.
J. Physiol. (Lond.),
2003
Apr
15
, 548 (353-60).
Chung HJ
et al.
Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains.
Proc. Natl. Acad. Sci. U.S.A.,
2006
Jun
6
, 103 (8870-5).
Schwake M
et al.
Surface expression and single channel properties of KCNQ2/KCNQ3, M-type K+ channels involved in epilepsy.
J. Biol. Chem.,
2000
May
5
, 275 (13343-8).
Etxeberria A
et al.
Three mechanisms underlie KCNQ2/3 heteromeric potassium M-channel potentiation.
J. Neurosci.,
2004
Oct
13
, 24 (9146-52).
Nakajo K
et al.
Second coiled-coil domain of KCNQ channel controls current expression and subfamily specific heteromultimerization by salt bridge networks.
J. Physiol. (Lond.),
2008
Jun
15
, 586 (2827-40).
Yang WP
et al.
Functional expression of two KvLQT1-related potassium channels responsible for an inherited idiopathic epilepsy.
J. Biol. Chem.,
1998
Jul
31
, 273 (19419-23).
Cooper EC
et al.
Colocalization and coassembly of two human brain M-type potassium channel subunits that are mutated in epilepsy.
Proc. Natl. Acad. Sci. U.S.A.,
2000
Apr
25
, 97 (4914-9).
Brown DA
et al.
Regulation of M(Kv7.2/7.3) channels in neurons by PIP(2) and products of PIP(2) hydrolysis: significance for receptor-mediated inhibition.
J. Physiol. (Lond.),
2007
Aug
1
, 582 (917-25).
Contributors: Rajnish Ranjan, Michael Schartner, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/25/ , accessed on 2026 Feb 23