Kir5.1
Description: potassium inwardly-rectifying channel, subfamily J, member 16 Gene: Kcnj16 Alias: Kir5.1, kcnj16, BIR9
Of the 80 different K+ channel genes found in the human genome, 15 belong to the family of inwardly-rectifying potassium (Kir) channels which are further subdivided into seven different classes (Kir1.1–Kir7.1) (Bichet[1041]). The Kir5.1 subunit does not form functional channels by itself and has no related homologs in the mammalian genome (Pessia [1042], Constas [1019]). However, Kir5.1 co-assembles with Kir4.1 to form novel heteromeric Kir4.1/Kir5.1 channels. (Shang [1040])
KCNJ16 (also known as BIR9; KIR5.1; MGC33717) encodes Kir5.1, an integral membrane protein, inward-rectifier type potassium channel, subfamily J, member 16. The encoded protein, which has a greater tendency to allow potassium to flow into a cell rather than out of a cell, can form heterodimers with two other inward-rectifier type potassium channels. It may be involved in the regulation of fluid and pH balance. Three transcript variants encoding the same protein have been found for this gene.
http://www.ncbi.nlm.nih.gov/gene/3773
Experimental data
Rat Kir5.1 gene in CHO host cells |
||
|
Click for details 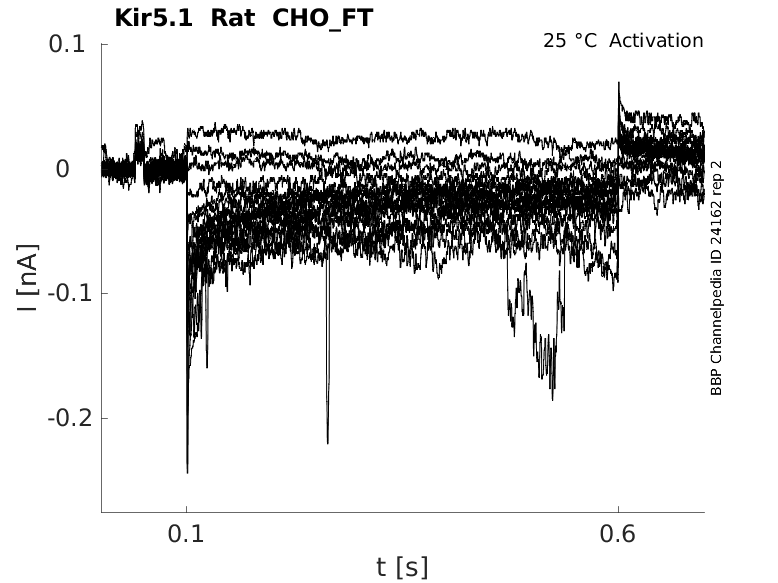
25 °Cshow 44 cells |
Click for details 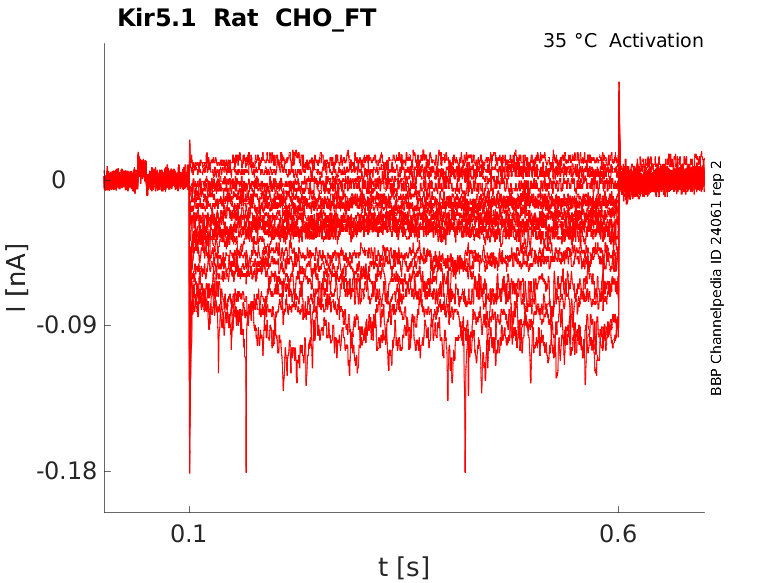
35 °Cshow 18 cells |
|
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_018658.4 | 4067 | |
| Mouse | NM_001252207.1 | 3718 | |
| Rat | NM_053314.3 | 1770 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Given the tetrameric nature of the K+ channel pore it is assumed that the central ion conduction pathway is not formed unless all four of the gating helices are in their ‘open’ conformation. Therefore, one structural model to explain the existence of K+ channel subconductance states is that these sublevels originate at the helix-bundle crossing due to successive movements of the four gating helices from the closed to open states, each movement producing a ‘partial’ opening of the channel on the way to the fully open state (Bezanilla [1044]). An alternative model proposed by Chapman & VanDongen suggests that the sublevels seen in the voltage-gated Kv2.1 channel originate from asymmetric conformations adopted by the selectivity filter in response to individual movements of the four gating helices (Chapman 2005 [1046]). Either way, both models assume that the allosteric interactions between identical subunits in a homomeric channel are highly cooperative, resulting in rapid transitions between the sublevels which are not resolved in the timescales of most single-channel recordings, making their analysis difficult, especially when obscured by noise and filtering. This behaviour, therefore, gives the appearance of a smooth and binary transition between the open and closed states (Bezanilla [1044], Chapman 2005 [1046]).
Kir5.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
The simplest models of ion channel gating are binary and alternate between two discrete permeation states: open and closed. The movement between these two states is thought to be controlled by a ‘gate’ which physically impedes the flow of ions in the closed state but which moves out of the way during the open state. However, such simple models of channel gating are challenged by the observation of intermediate conductance, or ‘subconductance’ levels, such as those seen in heteromeric Kir4.0/Kir5.1 channels as well as many other types of ion channel (Bezanilla [1044], Fox [1045], Chapman 2005 [1046], Chapman 1997 [1047]).
basolateral small conductance K+ channel in the distal nephron as well as pH-sensitive K+ channels in chemosensitive neurons (Pessia 1996 [1042], Pessia 2001 [1020]). Likewise, heteromeric Kir4.2/Kir5.1 channels have been reported in hepatic and pancreatic tissues (Pessia 2001 [1020], Pearson [205], Hill [1031]).
The functional properties of heteromeric Kir4.1/Kir5.1 channels are profoundly different to their parental subunits; homomeric Kir4.1 channels are only mildly sensitive to intracellular pH (IC50 ∼ 6.0) and have a single channel conductance of approximately 10 pS. By contrast, heteromeric Kir4.1/Kir5.1 channels are highly sensitive to intracellular pH (IC50 ∼ 6.8) and have a single channel conductance of ∼45 pS with multiple short-lived, subconductance states (Pessia 1996 [1042], Pessia 2001 [1020], Konstas [1019], Rapedius [1043], Tanemoto [1013], Giwa [1024], Xu [1023]).
References
Pearson WL
et al.
Expression of a functional Kir4 family inward rectifier K+ channel from a gene cloned from mouse liver.
J. Physiol. (Lond.),
1999
Feb
1
, 514 ( Pt 3) (639-53).
Tanemoto M
et al.
In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1.
J. Physiol. (Lond.),
2000
Jun
15
, 525 Pt 3 (587-92).
Konstas AA
et al.
Identification of domains that control the heteromeric assembly of Kir5.1/Kir4.0 potassium channels.
Am. J. Physiol., Cell Physiol.,
2003
Apr
, 284 (C910-7).
Pessia M
et al.
Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1.
J. Physiol. (Lond.),
2001
Apr
15
, 532 (359-67).
Xu H
et al.
Modulation of kir4.1 and kir5.1 by hypercapnia and intracellular acidosis.
J. Physiol. (Lond.),
2000
May
1
, 524 Pt 3 (725-35).
Cui N
et al.
Modulation of the heteromeric Kir4.1-Kir5.1 channels by P(CO(2)) at physiological levels.
J. Cell. Physiol.,
2001
Nov
, 189 (229-36).
Hill CE
et al.
Cloning, expression, and localization of a rat hepatocyte inwardly rectifying potassium channel.
Am. J. Physiol. Gastrointest. Liver Physiol.,
2002
Feb
, 282 (G233-40).
Paulais M
et al.
Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome.
Proc. Natl. Acad. Sci. U.S.A.,
2011
Jun
21
, 108 (10361-6).
Trapp S
et al.
Respiratory responses to hypercapnia and hypoxia in mice with genetic ablation of Kir5.1 (Kcnj16).
Exp. Physiol.,
2011
Apr
, 96 (451-9).
Wenker IC
et al.
Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism.
J. Neurophysiol.,
2010
Dec
, 104 (3042-52).
Shang L
et al.
Kir5.1 underlies long-lived subconductance levels in heteromeric Kir4.1/Kir5.1 channels from Xenopus tropicalis.
Biochem. Biophys. Res. Commun.,
2009
Oct
23
, 388 (501-5).
Bichet D
et al.
Merging functional studies with structures of inward-rectifier K(+) channels.
Nat. Rev. Neurosci.,
2003
Dec
, 4 (957-67).
Pessia M
et al.
Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels.
EMBO J.,
1996
Jun
17
, 15 (2980-7).
Rapedius M
et al.
Control of pH and PIP2 gating in heteromeric Kir4.1/Kir5.1 channels by H-Bonding at the helix-bundle crossing.
Channels (Austin),
2007 Sep-Oct
, 1 (327-30).
Bezanilla F
The origin of subconductance levels in voltage-gated K+ channels.
J. Gen. Physiol.,
2005
Aug
, 126 (83-6).
Chapman ML
et al.
K channel subconductance levels result from heteromeric pore conformations.
J. Gen. Physiol.,
2005
Aug
, 126 (87-103).
Chapman ML
et al.
Activation-dependent subconductance levels in the drk1 K channel suggest a subunit basis for ion permeation and gating.
Biophys. J.,
1997
Feb
, 72 (708-19).
Credits
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/52/ , accessed on 2026 Mar 01