Kv1.4
Description: potassium voltage-gated channel, shaker-related subfamily, member 4 Gene: Kcna4 Alias: Kv1.4, kcna4, kv4, RK3, RHK1, KCHAN
Kv1.4, encoded by the gene KCNA4, is a member of the potassium voltage-gated channel subfamily A. Kv1.2 is widely distributed in teh central nervous system. Derugaltion of Kv1.4 leads to hypertrophy and heart failure
Experimental data
Rat Kv1.4 gene in CHO host cells datasheet |
||
|
Click for details 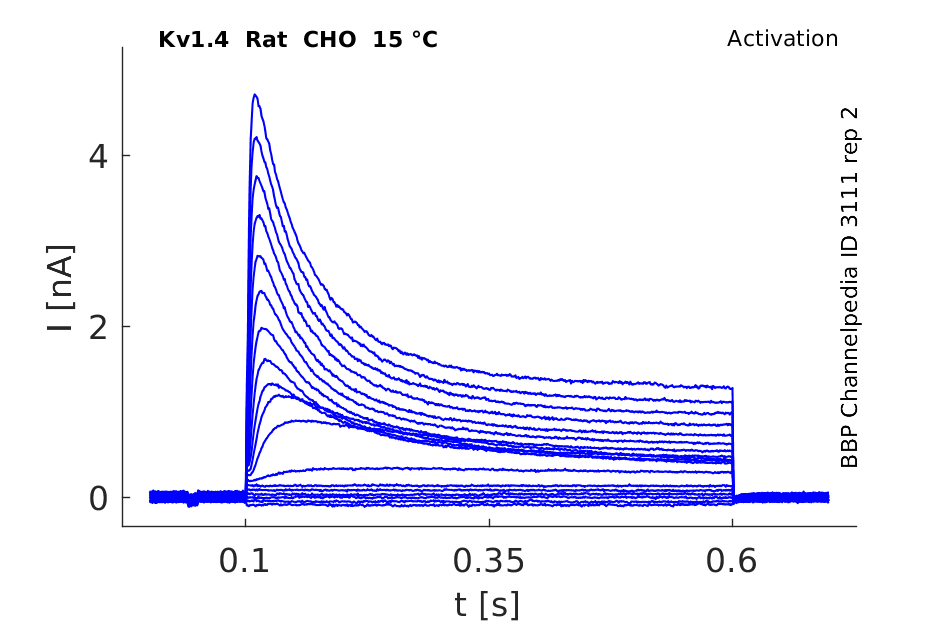
15 °Cshow 99 cells |
Click for details 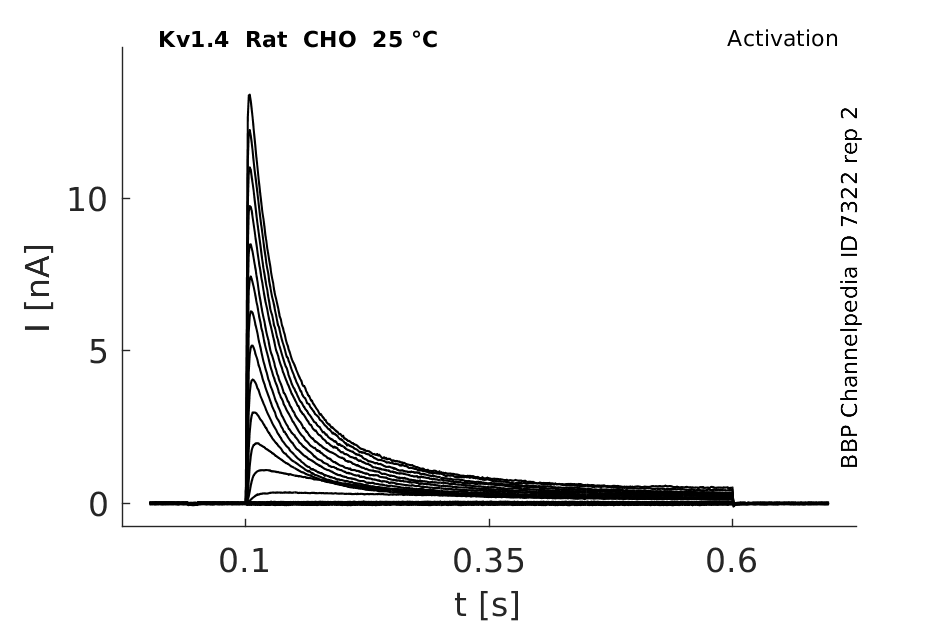
25 °Cshow 163 cells |
Click for details 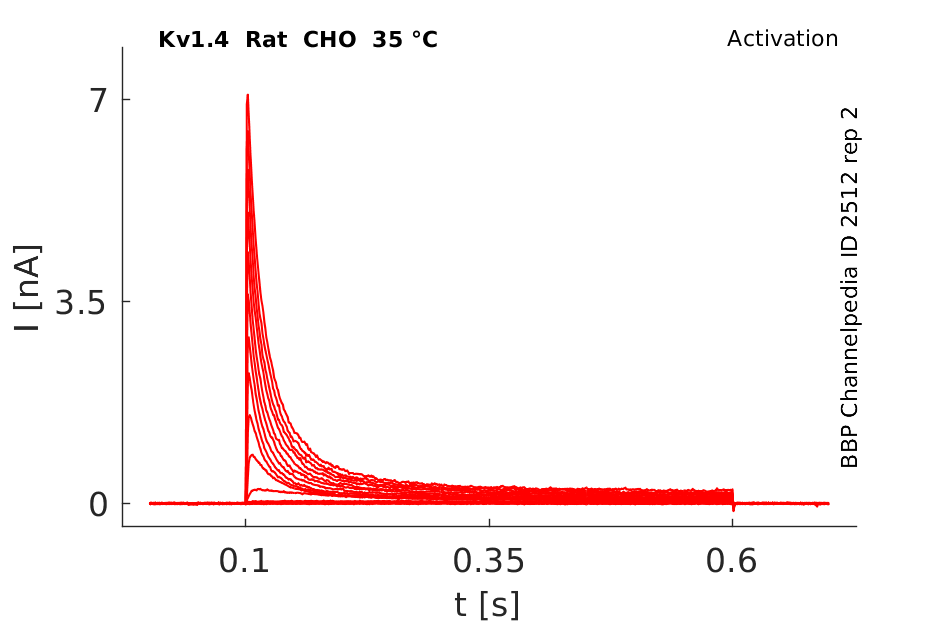
35 °Cshow 88 cells |
Mouse Kv1.4 gene in CHO host cells datasheet |
||
|
Click for details 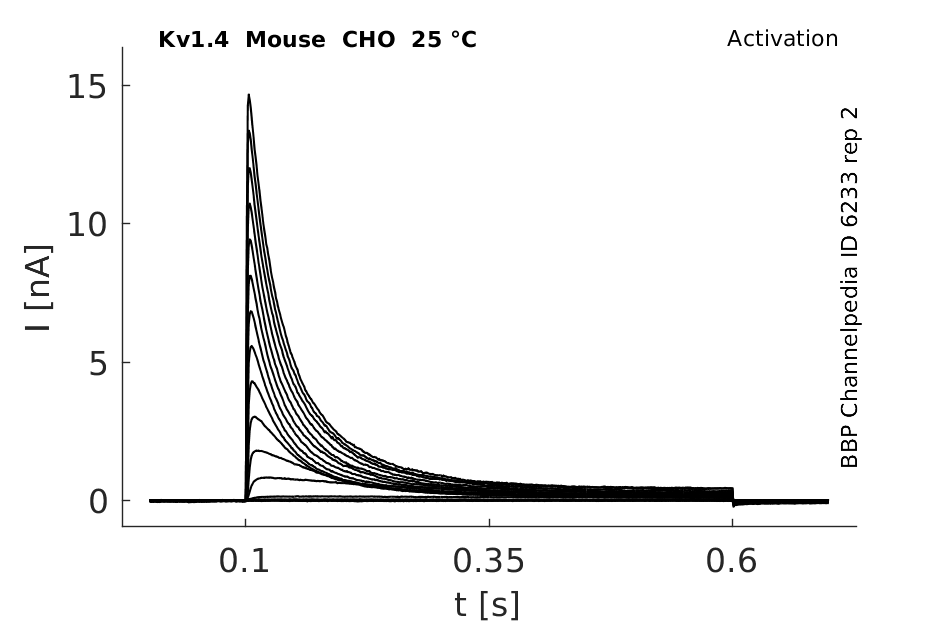
25 °Cshow 83 cells |
Click for details 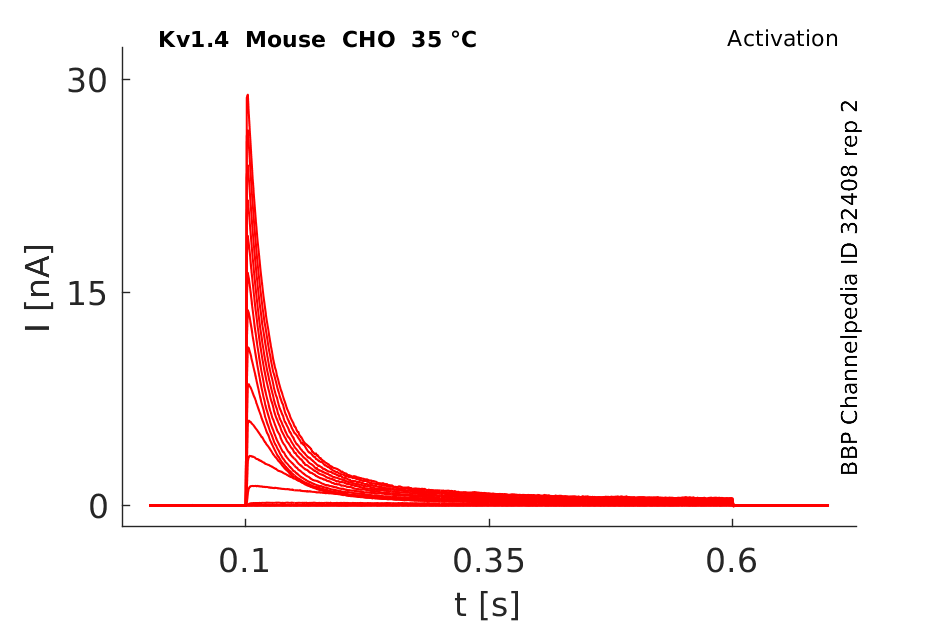
35 °Cshow 54 cells |
|
Human Kv1.4 gene in CHO host cells datasheet |
||
|
Click for details 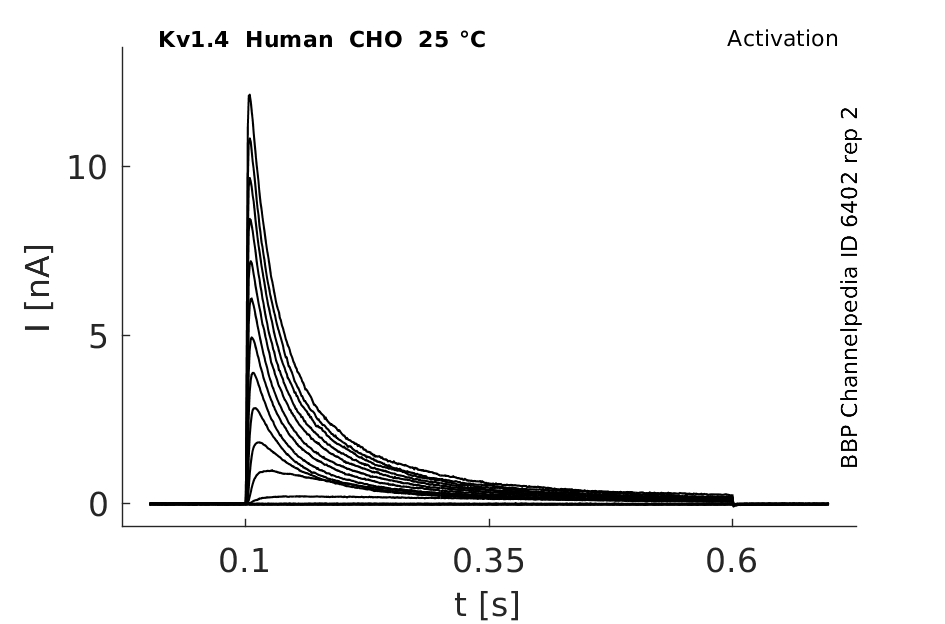
25 °Cshow 63 cells |
Click for details 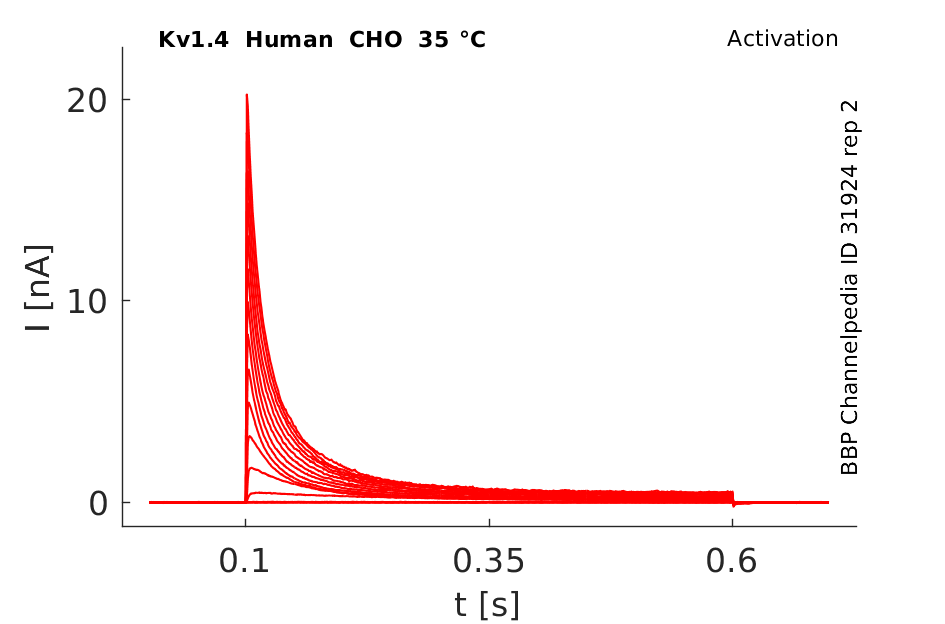
35 °Cshow 61 cells |
|
Rat Kv1.4 gene in HEK host cells datasheet |
||
|
Click for details 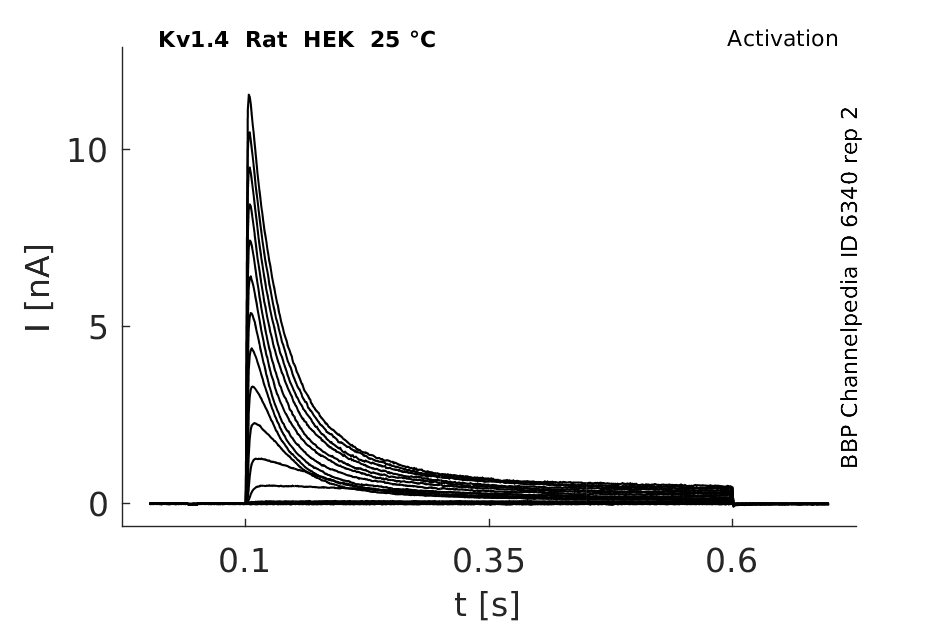
25 °Cshow 91 cells |
Click for details 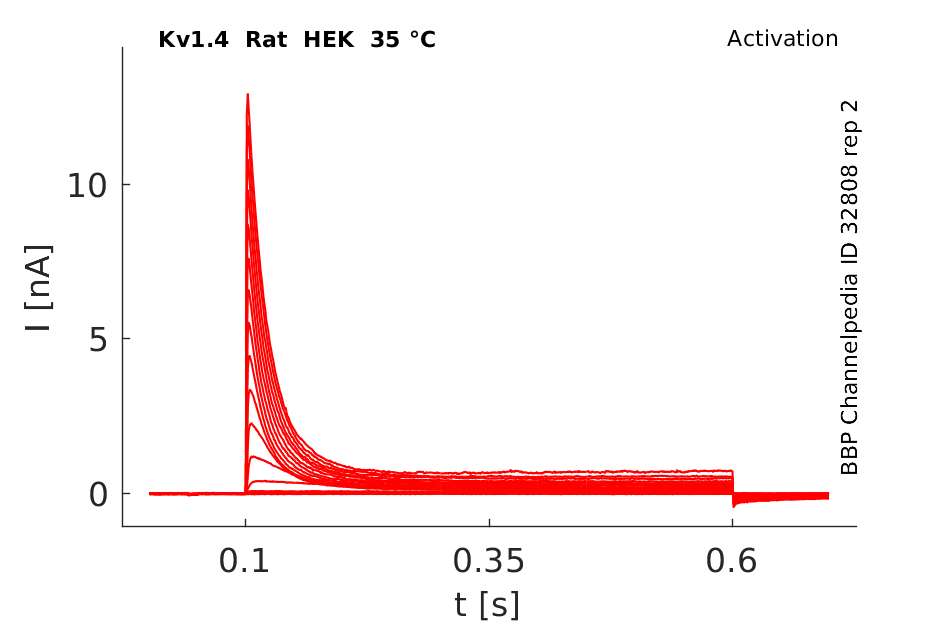
35 °Cshow 59 cells |
|
Rat Kv1.4 gene in CV1 host cells datasheet |
||
|
Click for details 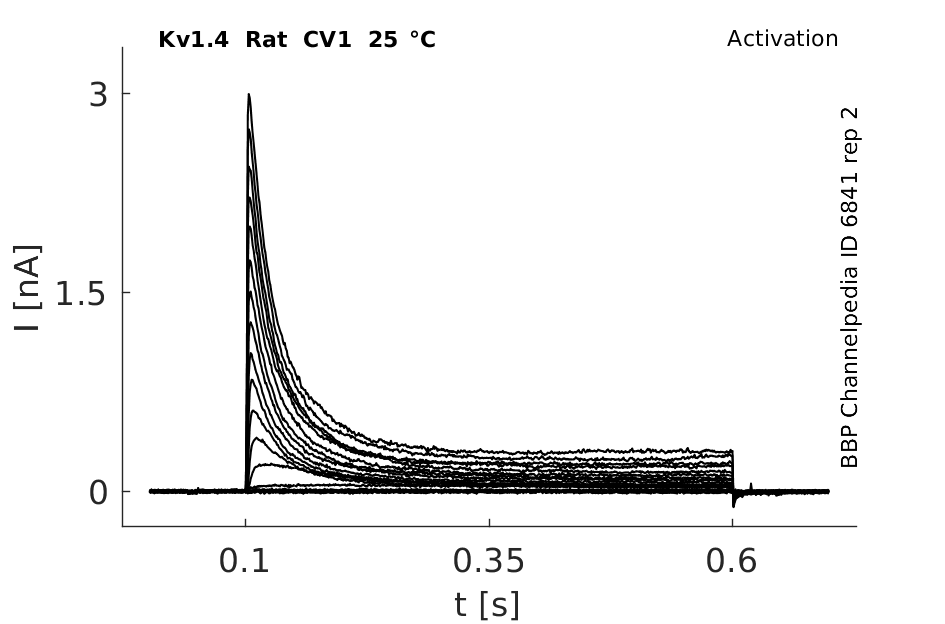
25 °Cshow 60 cells |
Click for details 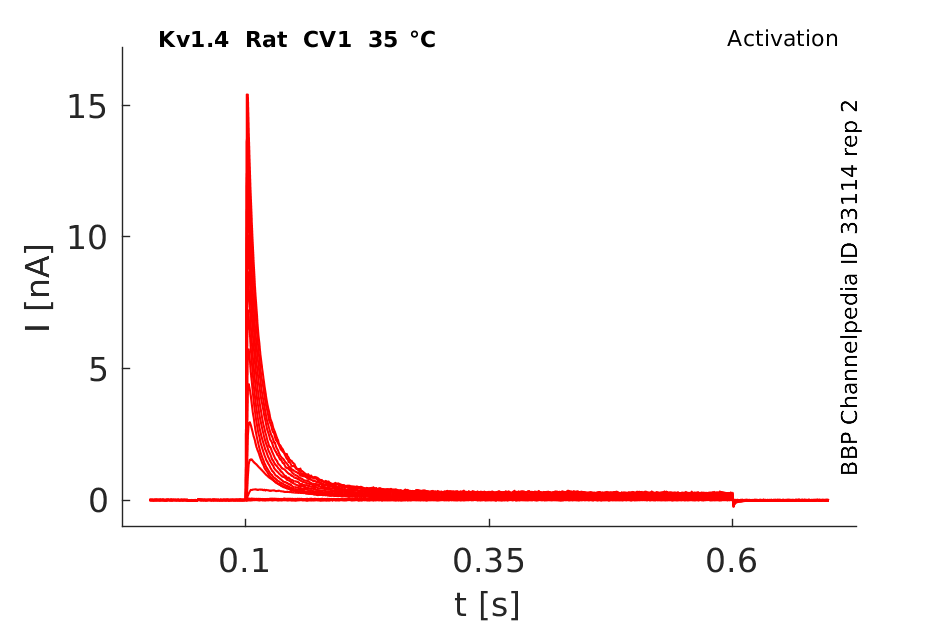
35 °Cshow 85 cells |
|
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_002233.4 | 4190 | |
| Mouse | NM_021275.4 | 4844 | |
| Rat | NM_012971.3 | 3127 |
Protein Isoforms
Isoforms
N-glycosylation
Kv1.4 channels are highly glycosylated. The composition of N-glycosylation was shown to affect the trafficking and cell surface expression levels of Kv1.4 channels [2016]
Visual Representation of Kv1.4 Structure
Methodology for visual representation of structure available here
Crystal Structure of Kv1.4
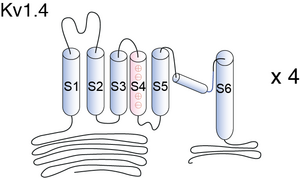
Kv1.4 is a typical voltage-gated channel with six transmembrane-spanning domains, one of which (S4) is charged and thought to be the voltage sensor. The N-terminal forms a ball and chain which can occlude the open channel. In the ΔN construct, amino acids 2–146 are deleted, removing the N-terminal and thus N-type inactivation. A lysine-to-tyrosine point mutation on the extracellular side of S6 was made (Kv1.4[K532Y]). This mutation drastically reduces the ability of the channel to undergo C-type inactivation [1611]
A model of the Kv1.4 pore, based on the crystal structure of KcsA, shows that H508 and K532 lie close together. It is suggested that the acidosis-induced increase of C-type inactivation involves the charge on H508 and K532 [1615]
Kv1.4 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Single Channel Current
The single-channel conduc- tance of Kv1.4 is much smaller than that of either Kv1.1 or Kv1.6. Ludewig et al 1993 presented current traces which suggested that the outer pore mutation K533Y increases the conductance of Kv1.4 channels at –60 mV. Our data support this finding; however,we have found that this mutation also produces a smaller but sig- nificant (P < 0.001) increase in open-channel conductance at +60 mV both in symmetrical high K+ and in asymmetrical K+. The conductance of Kv1.4 at +60 mV in symmetrical high K+ was increased from ap- prox. 7.8 pS to 10.5 pS
Kv1.4 ion channels undergo inactivation via two distinct mechanisms. N-type inactivation is fast, and, when present, dominates channel closure. C-type inactivation is slower, and is the rate-limiting step controlling the rate of recovery from inactivation. C-type inactivation therefore plays a crucial role in the availability of the channel for the next stimulation in physiological systems [1611]
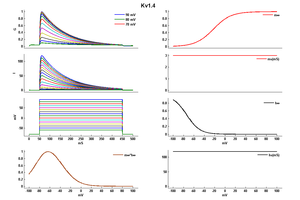
Kv1.4 Kinetics
Kv1.4 α subunits display: (i) rapid sigmoidal activation kinetics; (ii) biexponential inactivation kinetics; (iii) very slow kinetics of recovery (time constants on the order of seconds); (iv) cumulative inactivation during repetitive voltage clamp pulses; (v) open-state block by 4-AP, and (vi) insensitivity to HPTXs, PaTXs and flecanide [1617]
Kv1.4 in CHO cells is altered by KCNEs

Kv1.4 channels inactivation measured in Xenopus oocytes and effect of intracellular heme
K+ currents were measured using the inside-out patch-clamp method in Xenopus oocytes expressing Kv1.4. The inactivation constant was 48.2 ± 2.8ms. Application of hemin (200 nM) slowed down the inactivation of Kv1.4 channels to 245 ± 21 ms.[2014]
Kv1.4 inactivation in HEK293T cells and effect of synaptotagmin
Using the patch-clamp technique Kv1.4 currents were measured in HEK293T cells. The inactivation time of Kv1.4 channels was 38.1 ± 2.3 ms at a membrane potential of +30mV. Co expression of synaptotagmin I lead to the N-type fast inactivation of Kv1.4 being delayed to 68.8 ± 2.6 ms in Ca2+ dependent manner.[1609]
Model Kv1.4 (ID=20)
Kslow modified (V shift of -21mV)
| Animal | rat | |
| CellType | Oocyte | |
| Age | 0 Days | |
| Temperature | 0.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [274] O Pongs et. al; EMBO J. 1989 Nov | |
| mpower | 1.0 | |
| m Inf | 1.0000/(1+ exp((v - -21.7000)/-16.9000)) | |
| m Tau | 3.0000 | |
| hpower | 1.0 | |
| h Inf | 1.0000/(1+ exp((v - -73.6000)/12.8000)) | |
| h Tau | 119.0000 | |
Kv1.4 Expression in Brain
Kv1.4 subunit were localized in the AIS of cortical cells in P10 rats.[427]
Kv1.4's expression is time-dependent: The protein expression of the A-type (fast-inactivating) potassium channel Kv1.4 started around P10 in situ in granule cells of the DG, and in neurons of stratum pyramidale of the CA3 area with strongly labeled somata on P12. The expression peaked around P14 and diminished on P15, paralleled by the emerging appearance of Kv1.4-positive staining in the inner molecular layer of DG and in the mossy fiber tract.
Expression related to Kv co-localization
Kv1.1 and Kv1.4 are found robustly expressed in the relative absence of Kv1.2 within the striatal efferents in globus pallidus and pars reticulata of substantial nigra. Kv1.1, Kv1.2, and Kv1.4 are highly expressed in the middle third of the molecular layer of the dentate gyrus where they are associated with axons and terminals of the medial perforant path. Kv1.1, Kv1.2, and Kv1.4 are also found in Schaffer collateral axons, whereas Kv1.1 and Kv1.4 colocalize, in the absence of Kv1.2, in mossy fiber axons [1368].
The onset and development of Kv1.4 protein expression depend on neuronal activity in mouse hippocampal neurons.[378]
The strong expression of Kv1.4 protein in the adult mossy fiber tract underlines the important role in modulating electrophysiological behavior in the mouse hippocampus.[378]
Adrenal zona fasciculata
In the adrenal zona fasciculata Kv1.4, together with Cav3.2 and TREK-1 shape the cellular current properties[2013]
Distribution in Neuron
Kv1.4 are concentrated along axons and in the axonal membrane immediately preceding or within axon terminals. Immunoreactivity for Kv1.4 has been localized to the preterminal extensions of mossy fiber axons. In these positions, activation of Kv1 channels can play a critical role in regulating the extent of nerve terminal depolarization and thereby regulate neurotransmitter release. [1368]
Strong Kv1.4 signal in mossy fiber axons was seen between P16 and P17. In contrast to the Kv1.2 channel subunit, the strong expression of Kv1.4 in some interneurons of the CA3 area was restricted to their somatodendritic compartment as confirmed by primary cultures of hippocampal CA3 neurons. However, the axonal sorting of Kv1.4 was clearly visible in granule cells in primary cultures from DG. [378]
Kv1.4 Cellular Distribution in the Brain
Phosphorylation of voltage-gated K+ channels (Kv) is involved in regulation of neuronal excitability, synaptic plasticity and neuronal survival. Among Kv channels expressed in the CNS, Kv1.4 is located in the soma, dendrite and axon terminus of neurones in most regions of the brain [1802]
Function in the neuron
Although Kv1.4 contributes to a presynaptic A–type current to regulate neurotransmitter release [1609]
Fast inactivation of Kv1.4 is modulated by structural elements in the C-terminus.[10]
The expression of Kv1.4 in cortical cells of P10 rats converted the delayed rectifier Kv1.1/Kv1.2 channels to fast inactivating, transient A-type channels. [329]
Kv1.4 ion channels undergo inactivation via two distinct mechanisms. N-type inactivation is fast, and, when present, dominates channel closure. C-type inactivation is slower, and is the rate-limiting step controlling the rate of recovery from inactivation. C-type inactivation therefore plays a crucial role in the availability of the channel for the next stimulation in physiological systems [1611]
Spiking properties
Kv1.4 subunits contribute to the late-spiking (LS) properties displayed by small pyramidal neurons in layer 2/3 of rat retrosplenial cortex. These firing properties are a consequence of delayed rectifier and A-type potassium channels, identified as Kv1.4, as well as Kv1.1 and Kv4.3[1891]
Neuronal Excitability
A-type K+ channels encoded by Kv4.2, Kv4.3 and Kv1.4 differentially regulate intrinsic excitability of cortical pyramidal neurons [1798]
Disease
DIABETES
Diabetes slows down Ito recovery of inactivation because it triggers the switching from fast-recovering Kv4.x channels to the slow-recovering Kv1.4 [733]
HYPERTROPHY
The up-regulation of Kv1.4 expression could lead to hypertrophy and heart failure, indicating that the behavior of this channel is of particular clinical importance [1803]
BLADDER HYPER-EXCITABILITY
Results indicate that the excitability of capsaicin sensitive C-fiber bladder afferent neurons is increased in association with reductions in transient A-type K+ current density and Kv1.4 α-subunit expression in injured rats. Thus, the Kv1.4 α-subunit could be a molecular target for treating overactive bladder due to neurogenic detrusor overactivity [1613]
Alzheimer's
The Aβ peptide induces overexpression of Kv1.4 subunits in Alzheimer's. These expression levels could be attenuated in hippocampus and cortex by application of substance P through the cerebrospinal fluid. Indicating a possible neuroprotective activity of substance P[2011]
Myasthenia gravis
Cardiac auto-antibodies against Kv1.4 were implicated in myasthenia gravis [2012]
Circadian clock regulation
Membrane properties of neurons in the suprachiasmatic nucleus (SCN) were shown to feedback to regulate clock (PER2) expression. Kv1.4 and Kv4.2 channels may mediate this regulation of circadian pacemaker (PER2) and light-dependent responses [2015 ]
DPP10
Expressing a dipeptidyl peptidase-related ancillary subunit, DPP10, with Kv1.4 showed faster time to peak current, negative shifts in the half-inactivation potential of steady-state activation and inactivation but slower recovery from inactivation. (Many effects are the same as for DPP10 on Kv4.3) [9]
Propafenone
fKvl.4deltaN channel current was rapidly depressed in a frequency-dependent manner and C-type inactivation was strongly increased in the presence of 100 microM propafenone. While propafenone has no effect on Kv1.4deltaN recovery. [446]
Synaptotagmin I
Synaptotagmin I was found to delay the inactivation of Kv1.4 in HEK293T cells in a Ca2+-dependent manner, and this interaction was proven to have specificity. Mutagenesis experiments indicated that synaptotagmin I interacted with the N-terminus of Kv1.4 and thus delayed its N-type fast inactivation [1609]
Kv1.2
The Kv1.4/Kv1.2 heteromultimer combines features of both parent subunits, resulting in an A-type K+ channel. It has been proposed that both heteromultimers co assemble in the rat brain to regulate neurotransmitter release [733]
Kvβ1
Interestingly, the pattern of immunoreactivity for Kvβ1 closely matches the expression pattern for Kv1.1 and Kv1.4, in that Kvβ1 is found to colocalize with Kv1.4 in the medial perforant path, mossy fiber pathway, and in striatal efferents to the globus pallid us [1368]
Kvβ3
Kv beta 3 slowed recovery from inactivation for Kv1.4, but not for a Kv1.4 deletion mutant lacking N-type inactivation. Finally, steady-state activation and the time course of Kv1.4 current activation were not strongly influenced by Kv beta 3; however, deactivation was slowed in the presence of Kv beta 3 [1616]
Kvβ1.3
Kvβ1.3 accelerates both the fast and slow components of inactivation, promotes the slower component of inactivation and slows recovery of Kv1.4 [1617]
Quinidine
Quinidine is a commonly used anti-arrhythmic agent which blocks K+ channels. Quinidine block of the Kv1.4ΔN channel is a complex process, involving more than one conformational state. Initially, quinidine blocks the open channel with rapid kinetics on the same time scale as activation. Subsequently, the quinidine-bound channel enters a C-type inactivated state in a time- and voltage-dependent manner. Recovery from this state is the same as recovery from C-type inactivation, and governs the use dependence of quinidine block. The apparent dissociation constant for quinidine was reduced by increasing [K+]o to 98 mm (KD = 374.7 μm, n = 9) or a lysine to tyrosine mutation at position 532 [1614]
Arachidonic Acid
Arachidonic acid potently inhibits both postsynaptic-type Kv4.2 and presynaptic-type Kv1.4 IA potassium channels [1799]
Ginsenoside
We have demonstrated previously that the 20(S) but not the 20(R) form of ginsenoside Rg(3) inhibited K(+) currents flowing through Kv1.4 (hKv1.4) channels expressed in Xenopus laevis oocytes, pointing to the presence of specific interaction site(s) for Rg(3) in the hKv1.4 channel [1800]
Curcumin

Intracellular heme
Heme interacts with N-terminal inactivation domain of Kv1.4 channels. A process that enables intracellular free heme to potently impair Kv1.4 ball-and-chain inactivation [2014]
Heteromeric Interaction Kv1.4 and Kv1.1
The membrane potential of spiral ganglion neurons (SGN) of Kcna2 null mice was significantly hyperpolarized compared with that of wild-type SGNs. It was suggested that heteromultimerization of Kv1.2 and Kv1.4 α-subunits defines the properties of SGNs [1604]
Hydrogensulfate
Nociceptive processing in trigeminal ganglion (TG) neurons involves endogenous H2S generating enzyme (CBS) co-localization with Kv1.1 and Kv1.4. Application of NaHS, an H2S donor, supresses IK density with impact on neuronal excitability[1893]
References
Li HL
et al.
DPP10 is an inactivation modulatory protein of Kv4.3 and Kv1.4.
Am. J. Physiol., Cell Physiol.,
2006
Nov
, 291 (C966-76).
Sankaranarayanan K
et al.
N type rapid inactivation in human Kv1.4 channels: functional role of a putative C-terminal helix.
Mol. Membr. Biol.,
2005 Sep-Oct
, 22 (389-400).
Stühmer W
et al.
Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain.
EMBO J.,
1989
Nov
, 8 (3235-44).
Lorincz A
et al.
Cell-type-dependent molecular composition of the axon initial segment.
J. Neurosci.,
2008
Dec
31
, 28 (14329-40).
Prüss H
et al.
Age-dependent axonal expression of potassium channel proteins during development in mouse hippocampus.
,
2009
Dec
12
, ().
Fan Z
et al.
Electrostatic interaction in the NH(2)-terminus accelerates inactivation of the Kv1.4 channel.
Biochim. Biophys. Acta,
2010
Nov
, 1798 (2076-83).
Bodeker M
et al.
Position-dependent attenuation by Kv1.6 of N-type inactivation of Kv1.4-containing channels.
,
2011
Feb
25
, ().
Ogawa Y
et al.
Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2.
J. Neurosci.,
2008
May
28
, 28 (5731-9).
Jo A
et al.
Dendritic localization and a cis-acting dendritic targeting element of Kv4.2 mRNA.
BMB Rep,
2010
Oct
, 43 (677-82).
Amarillo Y
et al.
Ternary Kv4.2 channels recapitulate voltage-dependent inactivation kinetics of A-type K+ channels in cerebellar granule neurons.
J. Physiol. (Lond.),
2008
Apr
15
, 586 (2093-106).
Amarillo Y
et al.
Ternary Kv4.2 channels recapitulate voltage-dependent inactivation kinetics of A-type K+ channels in cerebellar granule neurons.
J. Physiol. (Lond.),
2008
Apr
15
, 586 (2093-106).
Tian L
et al.
Effect of propafenone on Kv1.4 inactivation.
J. Physiol. Biochem.,
2006
Dec
, 62 (263-70).
Baehring R
et al.
Mechanisms of Closed-State Inactivation in Voltage-Gated Ion Channels.
,
2010
Nov
22
, ().
Skerritt MR
et al.
Role of S4 positively charged residues in the regulation of Kv4.3 inactivation and recovery.
Am. J. Physiol., Cell Physiol.,
2007
Sep
, 293 (C906-14).
Wang S
et al.
Activation properties of Kv4.3 channels: time, voltage and [K+]o dependence.
J. Physiol. (Lond.),
2004
Jun
15
, 557 (705-17).
Hashimoto Y
et al.
Changes in the inactivation of rat Kv1.4 K(+) channels induced by varying the number of inactivation particles.
J. Biol. Chem.,
2000
Mar
31
, 275 (9358-62).
Clay JR
Determining K+ channel activation curves from K+ channel currents.
Eur. Biophys. J.,
2000
, 29 (555-7).
Judge SI
et al.
Inactivation gating and 4-AP sensitivity in human brain Kv1.4 potassium channel.
Brain Res.,
1999
Jun
12
, 831 (43-54).
Sheng M
et al.
Presynaptic A-current based on heteromultimeric K+ channels detected in vivo.
Nature,
1993
Sep
2
, 365 (72-5).
Trimmer JS
et al.
Localization of voltage-gated ion channels in mammalian brain.
Annu. Rev. Physiol.,
2004
, 66 (477-519).
Wang W
et al.
Association of the Kv1 family of K+ channels and their functional blueprint in the properties of auditory neurons as revealed by genetic and functional analyses.
J. Neurophysiol.,
2013
Oct
, 110 (1751-64).
Nashmi R
et al.
Abnormal axonal physiology is associated with altered expression and distribution of Kv1.1 and Kv1.2 K+ channels after chronic spinal cord injury.
Eur. J. Neurosci.,
2000
Feb
, 12 (491-506).
Gallego M
et al.
Transient outward potassium channel regulation in healthy and diabetic hearts.
Can. J. Physiol. Pharmacol.,
2009
Feb
, 87 (77-83).
Xie C
et al.
Synaptotagmin I delays the fast inactivation of Kv1.4 channel through interaction with its N-terminus.
Mol Brain,
2014
, 7 (4).
Bett GC
et al.
A model of the interaction between N-type and C-type inactivation in Kv1.4 channels.
Biophys. J.,
2011
Jan
5
, 100 (11-21).
Zhou Q
et al.
Markov models of use-dependence and reverse use-dependence during the mouse cardiac action potential.
PLoS ONE,
2012
, 7 (e42295).
Takahashi R
et al.
Hyperexcitability of bladder afferent neurons associated with reduction of Kv1.4 α-subunit in rats with spinal cord injury.
J. Urol.,
2013
Dec
, 190 (2296-304).
Wang S
et al.
Kv1.4 channel block by quinidine: evidence for a drug-induced allosteric effect.
J. Physiol. (Lond.),
2003
Jan
15
, 546 (387-401).
Claydon TW
et al.
Two pore residues mediate acidosis-induced enhancement of C-type inactivation of the Kv1.4 K(+) channel.
Am. J. Physiol., Cell Physiol.,
2002
Oct
, 283 (C1114-21).
Castellino RC
et al.
Time- and voltage-dependent modulation of a Kv1.4 channel by a beta-subunit (Kv beta 3) cloned from ferret ventricle.
Am. J. Physiol.,
1995
Jul
, 269 (H385-91).
Patel SP
et al.
Transient outward potassium current, 'Ito', phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms.
J. Physiol. (Lond.),
2005
Nov
15
, 569 (7-39).
Gómez-Hernandez JM
et al.
Molecular basis for different pore properties of potassium channels from the rat brain Kv1 gene family.
Pflugers Arch.,
1997
Nov
, 434 (661-8).
Kanda VA
et al.
KCNE1 and KCNE2 provide a checkpoint governing voltage-gated potassium channel α-subunit composition.
Biophys. J.,
2011
Sep
21
, 101 (1364-75).
Carrasquillo Y
et al.
A-type K+ channels encoded by Kv4.2, Kv4.3 and Kv1.4 differentially regulate intrinsic excitability of cortical pyramidal neurons.
J. Physiol. (Lond.),
2012
Aug
15
, 590 (3877-90).
Angelova PR
et al.
Arachidonic acid potently inhibits both postsynaptic-type Kv4.2 and presynaptic-type Kv1.4 IA potassium channels.
Eur. J. Neurosci.,
2009
May
, 29 (1943-50).
Lee JH
et al.
Ginsenoside Rg3 inhibits human Kv1.4 channel currents by interacting with the Lys531 residue.
Mol. Pharmacol.,
2008
Mar
, 73 (619-26).
Liu H
et al.
Curcumin potently blocks Kv1.4 potassium channels.
Biochem. Biophys. Res. Commun.,
2006
Jun
16
, 344 (1161-5).
Tao Y
et al.
Neuronal transmission stimulates the phosphorylation of Kv1.4 channel at Ser229 through protein kinase A1.
J. Neurochem.,
2005
Sep
, 94 (1512-22).
Guo W
et al.
Regulation of Kv4.2 and Kv1.4 K+ channel expression by myocardial hypertrophic factors in cultured newborn rat ventricular cells.
J. Mol. Cell. Cardiol.,
1998
Jul
, 30 (1449-55).
Roeper J
et al.
Frequency-dependent inactivation of mammalian A-type K+ channel KV1.4 regulated by Ca2+/calmodulin-dependent protein kinase.
J. Neurosci.,
1997
May
15
, 17 (3379-91).
Kurotani T
et al.
Pyramidal neurons in the superficial layers of rat retrosplenial cortex exhibit a late-spiking firing property.
Brain Struct Funct,
2013
Jan
, 218 (239-54).
Feng X
et al.
Hydrogen sulfide increases excitability through suppression of sustained potassium channel currents of rat trigeminal ganglion neurons.
Mol Pain,
2013
, 9 (4).
Campolongo P
et al.
Systemic administration of substance P recovers beta amyloid-induced cognitive deficits in rat: involvement of Kv potassium channels.
PLoS ONE,
2013
, 8 (e78036).
Suzuki S
et al.
Cardiac involvements in myasthenia gravis associated with anti-Kv1.4 antibodies.
Eur. J. Neurol.,
2014
Feb
, 21 (223-30).
Enyeart JJ
et al.
Ca2+ and K+ channels of normal human adrenal zona fasciculata cells: properties and modulation by ACTH and AngII.
J. Gen. Physiol.,
2013
Aug
, 142 (137-55).
Sahoo N
et al.
Heme impairs the ball-and-chain inactivation of potassium channels.
Proc. Natl. Acad. Sci. U.S.A.,
2013
Sep
30
, ().
Granados-Fuentes D
et al.
IA Channels Encoded by Kv1.4 and Kv4.2 Regulate Circadian Period of PER2 Expression in the Suprachiasmatic Nucleus.
J. Biol. Rhythms,
2015
Oct
, 30 (396-407).
Watanabe I
et al.
The degree of N-glycosylation affects the trafficking and cell surface expression levels of Kv1.4 potassium channels.
J. Membr. Biol.,
2015
Apr
, 248 (187-96).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/4/ , accessed on 2026 Feb 27