Cav3.3
Description: calcium channel, voltage-dependent, T type, alpha 1I subunit Gene: Cacna1i Alias: cacna1i, cav3.3, ca3.3, Ca(v)3.3
Cav3.3, encoded by the gene cacna1i, is a calcium channel, voltage-dependent, T type, alpha 1I subunit. It is highly expressed in the brain, where it is responsible for burst firing. Mutations of the channel are the cause of developmental disorders, particularly, schizophrenia.
In humans, cacna1i, the gene which encodes Cav3.3, is composed of 37 exons located on chromosome 22 at position 13. (22q13.1).
As with most Cav channels, cacna1i is subject to alternative splicing.
Some important exons, often involved in alternative splicing are [2525]:
- Exon 9: which adds 35 amino acids.
- Exon 33: Splicing within exon 33 leads to a 13 amino acid deletion (Δ33).
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_021096.4 | 10002 | |
| Mouse | NM_001044308.2 | 9833 | |
| Rat | NM_020084.3 | 6709 |
The human Cav3.3 protein is composed of 2223 amino acid (aa) and has a molecular weight of 245 Kda.
The CACNA1i gene undergoes extensive alternative splicing, leading to channels with subtle differences.
Cav3.3 isoforms lacking exon 9 (Δ9) recover faster from inactivation and show a steeper voltage dependence of inactivation compared to channels with exon 9. [2525]
Alternative splicing of exon 33, more specifically the region between exons 33 & 34, yields three key splice variants: Cav3.3Δ33, Cav3.3a, Cav3.3b, and Cav3.3c. These isoforms have variations in their carboxy-termini, leading to altered channel kinetics.
- Cav3.3a lacks the sequences found in b and c, and Exhibits the fastest activation and inactivation kinetics.
- Cav3.3b includes 40 extra nucleotides and has slower kinetics compared to Cav3.3a
- Cav3.3c includes 121 extra nucleotides and displays the slowest kinetics.
- Cav3.3Δ33 exhibits even slower activation and inactivation compared to their full-length counterparts [2526]
Isoforms
Little research has been conducted on Cav3.3 post translational modifications.
However, there is some evidence that phosphorylation regulates Cav3.3, particularly its activity-dependent Ca2+ inhibition behaviour [2487]
Some N-glycosylation sites have also been identified during structural studies [2527]
Like most Cav channels, Cav3.3 is made up of a single protein composed of 4 homologous domains (DI-DIV). Each domain is made up of 6 transmembrane subunits (S1-S6) connected by extracellular loops. S1-4 form the voltage sensing domain (VSD) whereas the S5-S6 act as the selectivity filter and forms the pore module (PM). The S4 subunit of each domain contains a series of positively charged residues. [2483]
The structure of a Cav3.3 isoform was resolved via electron-microscopy, to a resolution of 3.3 Å, allowing for a detailed insight into the specific structural features of the protein. [2527]:
- Extended S6 Helix in Domain III (S6III):
- A key structural feature of Cav3.3 is a long, bended S6 helix from domain III, extending into the cytosol. This extension is absent in other high-voltage-activated (HVA) calcium channels like CaV1 and CaV2.
- S6III helix has a positively charged cytoplasmic region (S6Cyto), which contributes to the channel's low-voltage activation properties. Mutations to this region shift the channel's voltage dependence, rendering iit less sensitive to low voltages.
- Unique Phospholipid Interactions:
- In the CaV3.3 channel, phospholipids penetrate the central cavity from domain interfaces, enhancing stability. Unlike CaV3.1, where phospholipids enter through the domain II–III fenestration, in CaV3.3, they enter from the domain III–IV interface, highlighting a structural difference that affects how these channels interact with their surrounding lipids
- Selectivity Filter and Ion Conduction:
- The selectivity filter of CaV3.3, like other calcium channels, is formed by the EEDD (glutamate, aspartate) motif, which controls calcium ion permeation.
- Gating Mechanism:
- CaV3.3 features slower kinetics, both in activation and inactivation, compared to other T-type channels such as CaV3.1 and CaV3.2, making it better suited for controlling sustained firing in neurons.
Cav3.3 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
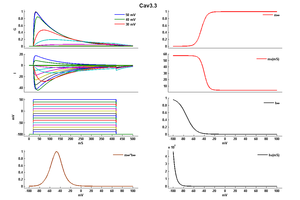
Cav3.3 T-channel current displayed slowly activating and inactivating kinetics. [112]
Cav3.3 by contrast is easily distinguishable by its slower activation and inactivation kinetics. [2528]
In exogenous systems the CaV3.1 and CaV3.2 T-types generally open and close at approximately similar membrane potentials, while CaV3.3 channels open and close at about +10 mV more depolarized potentials.9 Similarly, CaV3.1 and CaV3.2 channels inactivate (as determined at steady-state) around 5 mV more hyperpolarized than CaV3.3 channels and, therefore, require more hyperpolarized potentials to become available for subsequent opening via de-inactivation. CaV3.1 channels display the fastest activation and inactivation kinetics, marginally faster than CaV3.2, and both of which are significantly faster than for CaV3.3 channels.9,10 Therefore, CaV3.1 and CaV3.2 channels open fastest in response to depolarization when compared to CaV3.3, but are also quickest to inactivate during depolarization. Conversely, CaV3.3 channels display the fastest deactivation kinetics, far faster than CaV3.1, which is marginally faster than CaV3.2 and, therefore, upon repolarization CaV3.3 channels are the quickest to close when the membrane potential begins to repolarize. With respect to the rate at which the T-subtypes recover from inactivation, CaV3.1 channels display the fastest rate of recovery, followed by CaV3.3 and with CaV3.2 being the slowest. Experiments utilizing mock action potentials demonstrated that the CaV3.1 and CaV3.2 channels display similar amplitude currents in response to a single action potential, while due to the slower activation kinetics of CaV3.3 channels the resulting currents are significantly smaller.[2484]
Single channel unitary conductance
The single channel unitary conductance of Cav3.3 has not been measured as of yet.
Model
Model Cav3.3 (ID=42)
| Animal | CH | |
| CellType | CHO | |
| Age | 0 Days | |
| Temperature | 0.0°C | |
| Reversal | 30.0 mV | |
| Ion | Ca + | |
| Ligand ion | ||
| Reference | [103] Achraf Traboulsie et. al; J. Physiol. (Lond.) 2007 Jan 1 | |
| mpower | 1.0 | |
| m Inf | 1/(1+exp((v- -45.454426)/-5.073015)) | |
| m Tau | 3.394938 +( 54.187616 / (1 + exp((v - -40.040397)/4.110392))) | |
| hpower | 1.0 | |
| h Inf | 1 /(1+exp((v-(-74.031965))/8.416382)) | |
| h Tau | 109.701136 + (0.003816 * exp(-v/4.781719)) | |
Cav3.3 can be found in different locations of the body. However, it’s predominant highly expressed in the brain, particularly in the thalamus, particularly the nucleus reticularis thalami (nRt) [2529] [2415] [2530]
It has also been identified in other regions, such as:
Subcellular distribution of Cav3.3 depends on the localisation of the channel. In purkinje cells, the channel was predominantly identified in the dendrites and soma [2533]
Given its kinetics properties, Cav3.3 plays a role in sustained firing in neurons where it is expressed. In its predominant expression location, the reticular thalamic neurons (nRt), CaV3.3 channels are essential for generating rhythmic bursting which is critical for producing sleep spindles—a key feature of non-REM sleep. CaV3.3-mediated bursts, along with GluN2B-containing NMDA receptors, strengthen connections in thalamoreticular synapses, showing that sleep enhances intrathalamic circuits. Removing CaV3.3 channels in mice abolishes the low-frequency (4-10 Hz) oscillatory bursts required for sleep spindles, while regular tonic firing remains unaffected. This deletion also eliminates low-threshold calcium currents and spindles in the EEG, with further disruption seen when CaV3.2 channels are also removed, amplifying the negative effects on brain activity. These channels also regulate sleep spindle variability, influencing their frequency and amplitude across different brain regions. [2534] [2535] [2487] [2536]
CaV3.3 channels play a crucial role in mediating cerebral arterial constriction at lower intraluminal pressures [2532]
Channelopathies
Given the importance of Cav3.3’s roles, mutations to coding gene or disruption to the protein are responsible for a number of pathologies.
GWAS (genome-wide association studies) have identified genome-wide significant associations at loci including cacna1c and cacna1i for schizophrenia [2537] [2538] [2539] Schizophrenia is defined by waking phenomena, of which abnormal sleep is a common feature. Growing evidence points to the cause being a sleep spindle deficit. As previously discussed, Cav3.3 is largely present in these sleep spindles and is responsible for the regulation of their burst firing. [2530]
Cav3.3 mutation are associated with other neurodevelopmental disorders. Mutations in the channel gate region result in slowed channel kinetics, altered voltage sensitivity, and increased calcium influx, leading to neuron hyperexcitability. These changes likely cause calcium toxicity, contributing to the patients' cognitive impairments and seizures [2540]. Some pathologies include:
CaV3.3 (cacna1i) is also notably under-expressed in both brain tumors and breast cancer [2440]. Additionally, certain mutations in cacna1i are linked to more aggressive breast cancer types, including invasive breast carcinoma stroma and ductal carcinoma in situ, suggesting that altered CaV3.3 calcium transport function may contribute to cancer progression and cellular dysregulation [2422]
So far no auxiliary subunits were shown to interact the activity of Cav3.3. however a number of other molecules and compounds have been demonstrated to modulate the channel:
- Zinc: slows both the inactivation and deactivation kinetics of Cav3.3. However, the Cav3.3 is 100 fold less sensitive to Zinc in comparison to other T-type channels. [103]
- Nickel, Niflumic acid, and mibefradil are well established blockers of Cav3.3 [2415]
- N-arachidonoyl glycine (NAGly) is a reversible inhibitor of Cav3.3 [2542]
- Phorbol-12-myristate-13-acetate (PMA) can enhance the current amplitude of cav3.3 without disruption the whole cell current [112]
References
Traboulsie A
et al.
Subunit-specific modulation of T-type calcium channels by zinc.
J. Physiol. (Lond.),
2007
Jan
1
, 578 (159-71).
Park JY
et al.
Activation of protein kinase C augments T-type Ca2+ channel activity without changing channel surface density.
J. Physiol. (Lond.),
2006
Dec
1
, 577 (513-23).
Park JY
et al.
Multiple structural elements contribute to the slow kinetics of the Cav3.3 T-type channel.
J. Biol. Chem.,
2004
May
21
, 279 (21707-13).
T-type Ca2+ channels encode prior neuronal activity as modulated recovery rates.
J. Physiol. (Lond.), 2006 Mar 15 , 571 (519-36).
Cataldi M
et al.
Zn(2+) slows down Ca(V)3.3 gating kinetics: implications for thalamocortical activity.
J. Neurophysiol.,
2007
Oct
, 98 (2274-84).
Kovacs K
et al.
Subcellular distribution of low-voltage activated T-type Ca2+ channel subunits (Ca(v)3.1 and Ca(v)3.3) in reticular thalamic neurons of the cat.
J. Neurosci. Res.,
2010
Feb
1
, 88 (448-60).
Randall AD
et al.
Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels.
Neuropharmacology,
1997
Jul
, 36 (879-93).
Perez-Reyes E
Molecular physiology of low-voltage-activated t-type calcium channels.
Physiol. Rev.,
2003
Jan
, 83 (117-61).
Armstrong CM
et al.
Two distinct populations of calcium channels in a clonal line of pituitary cells.
Science,
1985
Jan
4
, 227 (65-7).
Carbone E
et al.
A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones.
Nature,
1984 Aug 9-15
, 310 (501-2).
Perez-Reyes E
et al.
Molecular characterization of a neuronal low-voltage-activated T-type calcium channel.
Nature,
1998
Feb
26
, 391 (896-900).
Cribbs LL
et al.
Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family.
Circ. Res.,
1998
Jul
13
, 83 (103-9).
Lee JH
et al.
Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family.
J. Neurosci.,
1999
Mar
15
, 19 (1912-21).
Talley EM
et al.
Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels.
J. Neurosci.,
1999
Mar
15
, 19 (1895-911).
Kim D
et al.
Thalamic control of visceral nociception mediated by T-type Ca2+ channels.
Science,
2003
Oct
3
, 302 (117-9).
Ikeda H
et al.
Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia.
Science,
2003
Feb
21
, 299 (1237-40).
Shin JB
et al.
A T-type calcium channel required for normal function of a mammalian mechanoreceptor.
Nat. Neurosci.,
2003
Jul
, 6 (724-30).
Kawai F
et al.
Enhancement by T-type Ca2+ currents of odor sensitivity in olfactory receptor cells.
J. Neurosci.,
2001
May
15
, 21 (RC144).
Kessi M
et al.
Calcium channelopathies and intellectual disability: a systematic review.
Orphanet J Rare Dis, 2021May13, 16 (219).
Wang CY
et al.
Meta-Analysis of Public Microarray Datasets Reveals Voltage-Gated Calcium Gene Signatures in Clinical Cancer Patients.
PLoS ONE,
2015
, 10 (e0125766).
Phan NN
et al.
Voltage-gated calcium channels: Novel targets for cancer therapy.
Oncol Lett, 2017Aug, 14 (2059-2074).
Liao X
et al.
Genetic associations between voltage-gated calcium channels and autism spectrum disorder: a systematic review.
Mol Brain, 2020Jun22, 13 (96).
Cain SM
et al.
Contributions of T-type calcium channel isoforms to neuronal firing.
Channels (Austin),
2010 Nov-Dec
, 4 (475-82).
Lory P
et al.
Neuronal Cav3 channelopathies: recent progress and perspectives.
Pflugers Arch, 2020Jul, 472 (831-844).
Wang R
et al.
The Cav3.2 T-type calcium channel regulates temporal coding in mouse mechanoreceptors.
J. Physiol. (Lond.),
2011
May
1
, 589 (2229-43).
Murbartián J
et al.
Functional impact of alternative splicing of human T-type Cav3.3 calcium channels.
J. Neurophysiol.,
2004
Dec
, 92 (3399-407).
Murbartián J
et al.
Alternative splicing of the rat Ca(v)3.3 T-type calcium channel gene produces variants with distinct functional properties(1).
FEBS Lett.,
2002
Sep
25
, 528 (272-8).
He L
et al.
Structure, gating, and pharmacology of human CaV3.3 channel.
Nat Commun, 2022Apr19, 13 (2084).
Blanks AM
et al.
Characterization of the molecular and electrophysiological properties of the T-type calcium channel in human myometrium.
J. Physiol. (Lond.),
2007
Jun
15
, 581 (915-26).
Astori S
et al.
Synaptic plasticity at intrathalamic connections via CaV3.3 T-type Ca2+ channels and GluN2B-containing NMDA receptors.
J. Neurosci.,
2013
Jan
9
, 33 (624-30).
Manoach DS
et al.
Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition?
Biol Psychiatry, 2016Oct15, 80 (599-608).
Sharma A
et al.
Voltage-Gated T-Type Calcium Channel Modulation by Kinases and Phosphatases: The Old Ones, the New Ones, and the Missing Ones.
Cells, 2023Jan31, 12 ().
Harraz OF
et al.
CaV1.2/CaV3.x channels mediate divergent vasomotor responses in human cerebral arteries.
J. Gen. Physiol.,
2015
May
, 145 (405-18).
Masoli S
et al.
Action potential processing in a detailed Purkinje cell model reveals a critical role for axonal compartmentalization.
Front Cell Neurosci, 2015, 9 (47).
Astori S
et al.
The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus.
Proc. Natl. Acad. Sci. U.S.A.,
2011
Aug
16
, 108 (13823-8).
Fernandez LM
et al.
Thalamic reticular control of local sleep in mouse sensory cortex.
Elife, 2018Dec25, 7 ().
Pellegrini C
et al.
Suppression of Sleep Spindle Rhythmogenesis in Mice with Deletion of CaV3.2 and CaV3.3 T-type Ca(2+) Channels.
Sleep, 2016Apr01, 39 (875-85).
Heyne HO
et al.
Predicting functional effects of missense variants in voltage-gated sodium and calcium channels.
Sci Transl Med, 2020Aug12, 12 ().
Genome-wide association study implicates HLA-C*01:02 as a risk factor at the major histocompatibility complex locus in schizophrenia.
Biol. Psychiatry,
2012
Oct
15
, 72 (620-8).
Baez-Nieto D
et al.
Analysing an allelic series of rare missense variants of CACNA1I in a Swedish schizophrenia cohort.
Brain, 2022Jun03, 145 (1839-1853).
El Ghaleb Y
et al.
CACNA1I gain-of-function mutations differentially affect channel gating and cause neurodevelopmental disorders.
Brain, 2021Aug17, 144 (2092-2106).
Sanchez-Roige S
et al.
Genome-Wide Association Studies of Impulsive Personality Traits (BIS-11 and UPPS-P) and Drug Experimentation in up to 22,861 Adult Research Participants Identify Loci in the CACNA1I and CADM2 genes.
J Neurosci, 2019Mar27, 39 (2562-2572).
Barbara G
et al.
T-type calcium channel inhibition underlies the analgesic effects of the endogenous lipoamino acids.
J. Neurosci.,
2009
Oct
21
, 29 (13106-14).
Contributors: Rajnish Ranjan, Michael Schartner
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/87/ , accessed on 2026 Feb 25