Kv6.4
Description: potassium voltage-gated channel, subfamily G, member 4 Gene: Kcng4 Alias: Kv6.4, kcng4
Kv6.4 (also known as KV6.4; MGC4558; MGC129609), encoded by the gene KCNG4, is a member is a gamma subunit of the voltage-gated potassium channel, subfamily G. Kv6.4is electrically silent as it is not capable of forming function homotetrameric channels. It can heterotetramerize with Kv2.1 α-subunits to form functional Kv2.1/Kv6.4 channel complexes and is predominantly found in the brain NCBI
Experimental data
Rat Kv6.4 gene in CHO host cells datasheet |
||
|
Click for details 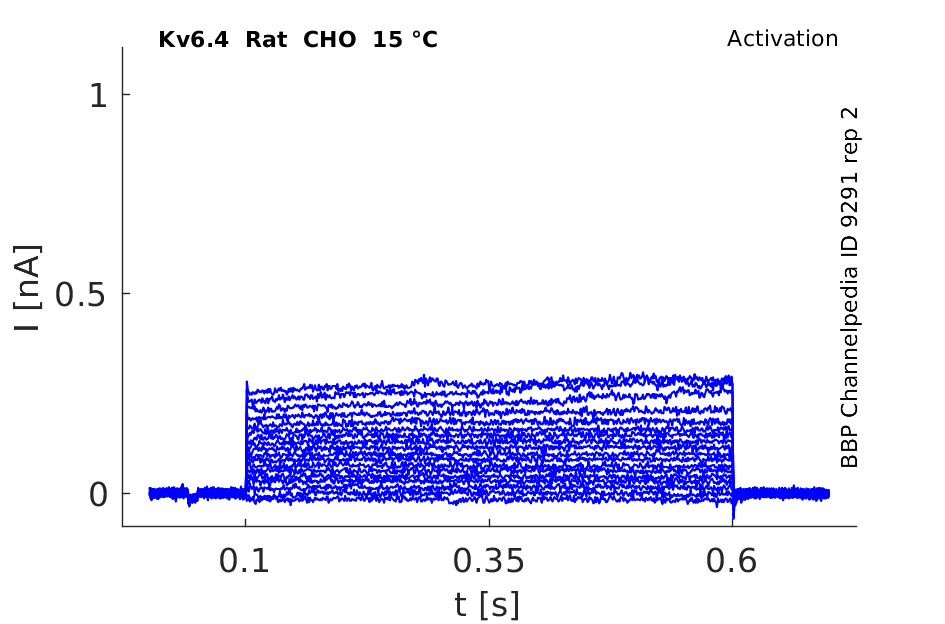
15 °Cshow 54 cells |
Click for details 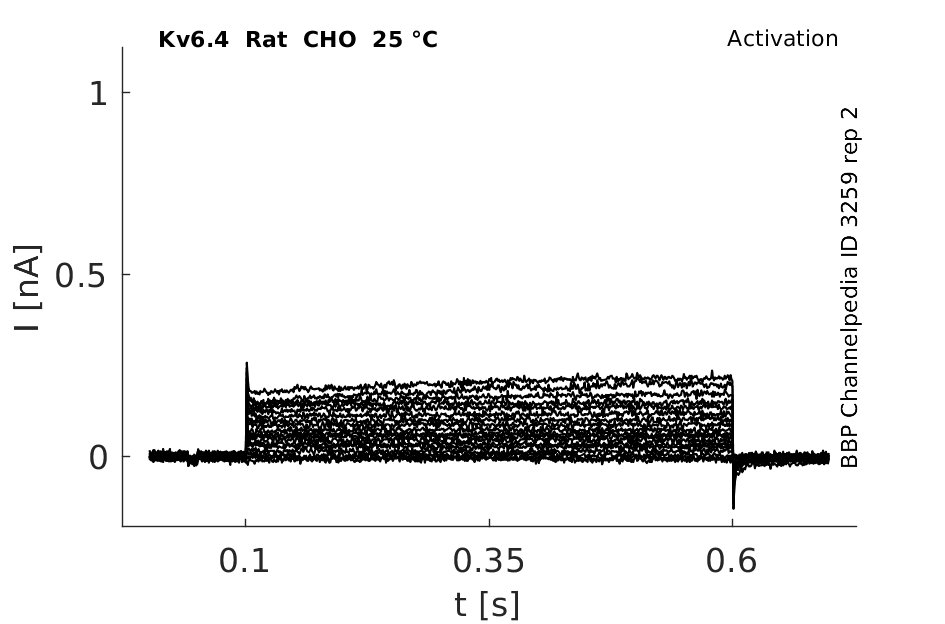
25 °Cshow 42 cells |
Click for details 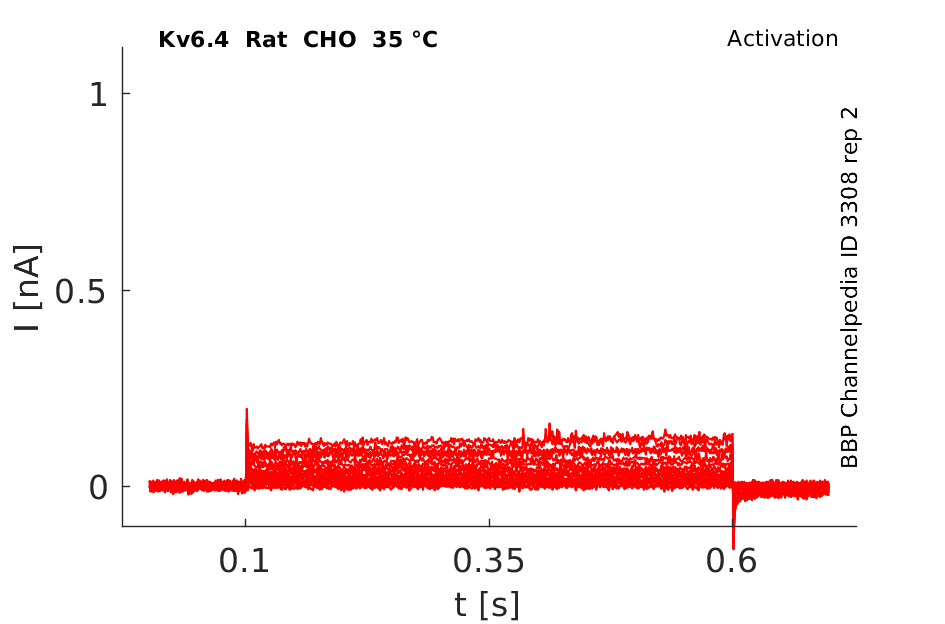
35 °Cshow 58 cells |
Mouse Kv6.4 gene in CHO host cells datasheet |
||
|
Click for details 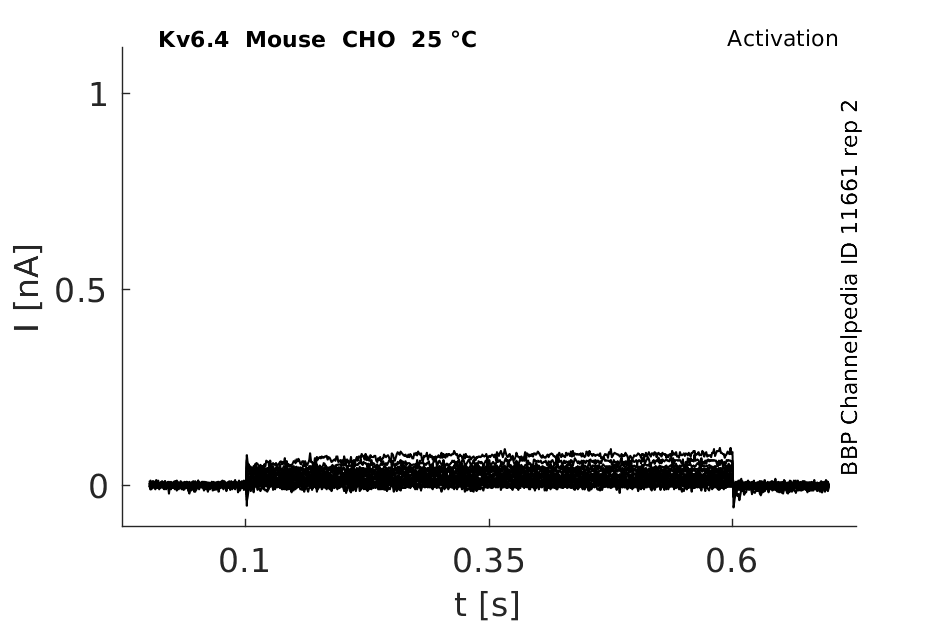
25 °Cshow 32 cells |
||
Human Kv6.4 gene in CHO host cells datasheet |
||
|
Click for details 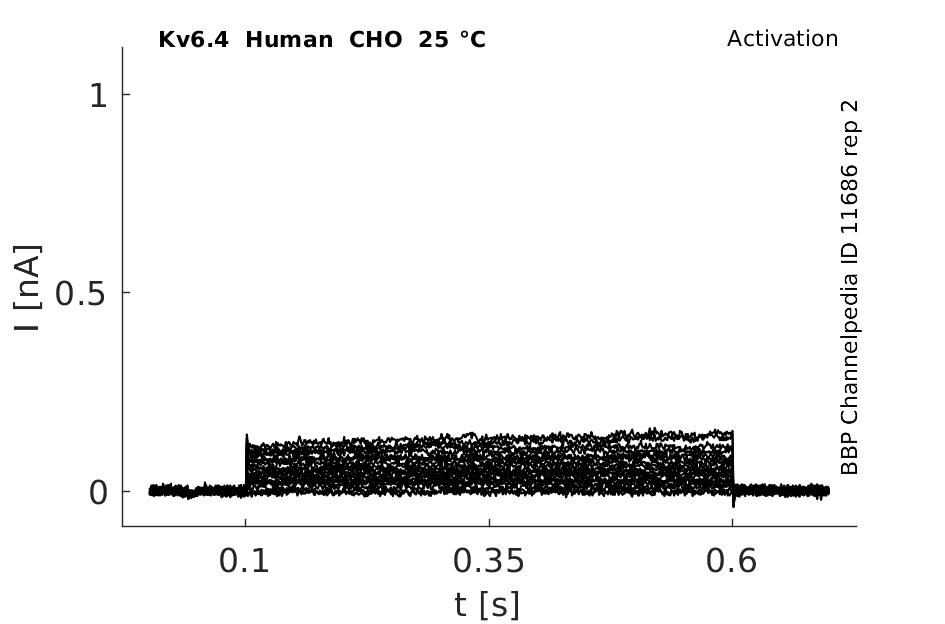
25 °Cshow 32 cells |
||
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_172347.3 | 5503 | |
| Mouse | NM_025734.2 | 3822 | |
| Rat | NM_001107435.1 | 4083 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv6.4 Structure
Methodology for visual representation of structure available here
Eight different voltage-gated K+ (Kv)3 Shaker-related channel subfamilies (Kv1–Kv6 and Kv8–Kv9) have been identified based on the degree of sequence homology [606]. Fully assembled Kv channels are composed of four α-subunits arranged around a central pore. Each α-subunit consists of six transmembrane segments S1–S6 with a cytoplasmic N and C terminus. The N terminus contains the T1 domain, a tetramerization domain that facilitates the assembly of α-subunits into functional channels. The presence of a T1 domain is not absolutely required for channel assembly because subunits without a T1 domain could also assemble into a functional tetramer, although less efficiently [677], [678], [679]. However, the T1 domain not only promotes but also restricts the formation of possible homo- and heterotetramers by preventing incompatible subunits from assembling [680], [660]. When four compatible T1 domains assemble, they are arranged with the same 4-fold symmetry as the transmembrane segments, forming a hanging gondola structure [681].
Kv6.4 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Human Kv6.4 Kinetics with Rat Kv2.1 in HEK293 Cells

Kv6.4 with Kv2.1 in HEK293 Cells

Markov Model for Kv2.1/Kv6.4 Channel Gating

Domains Required for Kv6.4 Function
It has been demonstrated that Kv2.1 chimeras containing either S1 or S5 from Kv6.4 were functional, wheras a chimeric Kv2.1 subunit containing both the Kv6.4 S1 and S5 segments did not form functional channels. However, back mutation of some residues in this S1/S5 chimera restored functionality, and it was shown that interactions between S1, S4, and S5 are important for the functionality of WT Kv2.1 (4). It is possible that the inability to form electrically functional channels in homotetrameric configuration is due to the lack of such S1/S4/S5 interactions, at least in the case of Kv6.4 [1840]
His-105 in the T1 domain of Kv2.1 is required for functional heteromerization with members of the Kv6 subfamily, such as Kv6.4. [664]
Migraine
Several genes encoding potassium channels, including KCNK18, KCNG4, and KCNAB3, were identified as potentially linked to migraine [1839]
Aspartates in the T1 domain are required for efficient assembly of both homotetrameric Kv2.1 and heterotetrameric Kv2.1/silent Kv6.4 channels. [677]
References
Shen NV
et al.
Molecular recognition and assembly sequences involved in the subfamily-specific assembly of voltage-gated K+ channel subunit proteins.
Neuron,
1995
Mar
, 14 (625-33).
Gutman GA
et al.
International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels.
Pharmacol. Rev.,
2005
Dec
, 57 (473-508).
Xu J
et al.
Assembly of voltage-gated potassium channels. Conserved hydrophilic motifs determine subfamily-specific interactions between the alpha-subunits.
J. Biol. Chem.,
1995
Oct
20
, 270 (24761-8).
Mederos Y Schnitzler M
et al.
Mutation of histidine 105 in the T1 domain of the potassium channel Kv2.1 disrupts heteromerization with Kv6.3 and Kv6.4.
J. Biol. Chem.,
2009
Feb
13
, 284 (4695-704).
Tu L
et al.
Voltage-gated K+ channels contain multiple intersubunit association sites.
J. Biol. Chem.,
1996
Aug
2
, 271 (18904-11).
Bocksteins E
et al.
Conserved negative charges in the N-terminal tetramerization domain mediate efficient assembly of Kv2.1 and Kv2.1/Kv6.4 channels.
J. Biol. Chem.,
2009
Nov
13
, 284 (31625-34).
Kobertz WR
et al.
K+ channels lacking the 'tetramerization' domain: implications for pore structure.
Nat. Struct. Biol.,
1999
Dec
, 6 (1122-5).
Zerangue N
et al.
An artificial tetramerization domain restores efficient assembly of functional Shaker channels lacking T1.
Proc. Natl. Acad. Sci. U.S.A.,
2000
Mar
28
, 97 (3591-5).
Lee TE
et al.
Structural determinant for assembly of mammalian K+ channels.
Biophys. J.,
1994
Mar
, 66 (667-73).
Kobertz WR
et al.
Hanging gondola structure of the T1 domain in a voltage-gated K(+) channel.
Biochemistry,
2000
Aug
29
, 39 (10347-52).
Bocksteins E
et al.
The electrically silent Kv6.4 subunit confers hyperpolarized gating charge movement in Kv2.1/Kv6.4 heterotetrameric channels.
PLoS ONE,
2012
, 7 (e37143).
Lafrenière RG
et al.
Identification of novel genes involved in migraine.
Headache,
2012
Oct
, 52 Suppl 2 (107-10).
Bocksteins E
et al.
Electrically silent Kv subunits: their molecular and functional characteristics.
Physiology (Bethesda),
2012
Apr
, 27 (73-84).
Bocksteins E
et al.
The subfamily-specific interaction between Kv2.1 and Kv6.4 subunits is determined by interactions between the N- and C-termini.
PLoS ONE,
2014
, 9 (e98960).
Contributors: Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/22/ , accessed on 2026 Feb 19