Kir2.3
Description: potassium inwardly-rectifying channel, subfamily J, member 4 Gene: Kcnj4 Alias: Kir2.3, HIR, HRK1, IRK3, HIRK2
One class of potassium channels is activated by depolarization whereas a second class is not. The latter are referred to as inwardly rectifying K+ channels, and they have a greater tendency to allow potassium to flow into the cell rather than out of it. This asymmetry in potassium ion conductance plays a key role in the excitability of muscle cells and neurons. The protein encoded by KCNJ4 (also known as HIR; HRK1; IRK3; HIRK2; IRK-3; Kir2.3; MGC142066; MGC142068) encodes the potassium inwardly-rectifying channel, subfamily J, member 4, named Kir2.3, which is an integral membrane protein. The encoded protein has a small unitary conductance compared to other members of this protein family. Two transcript variants encoding the same protein have been found for this gene. http://www.ncbi.nlm.nih.gov/gene/3761
Experimental data
Rat Kir2.3 gene in CHO host cells |
||
|
Click for details 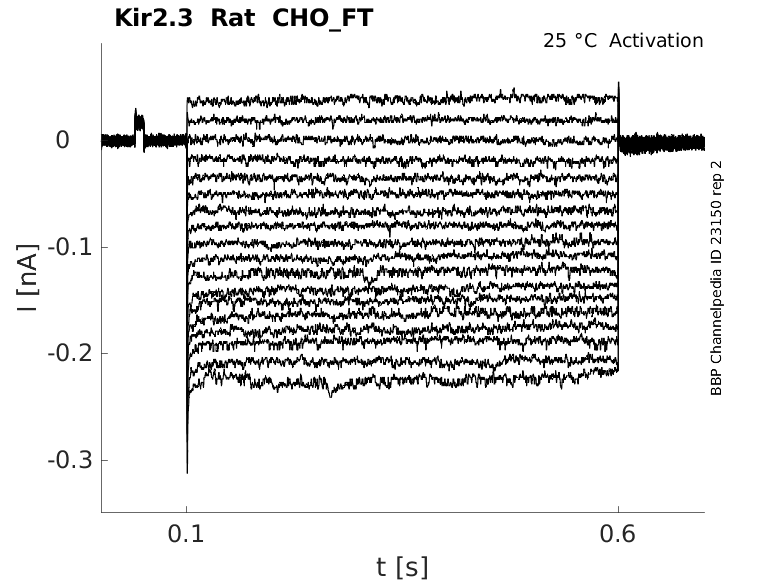
25 °Cshow 25 cells |
Click for details 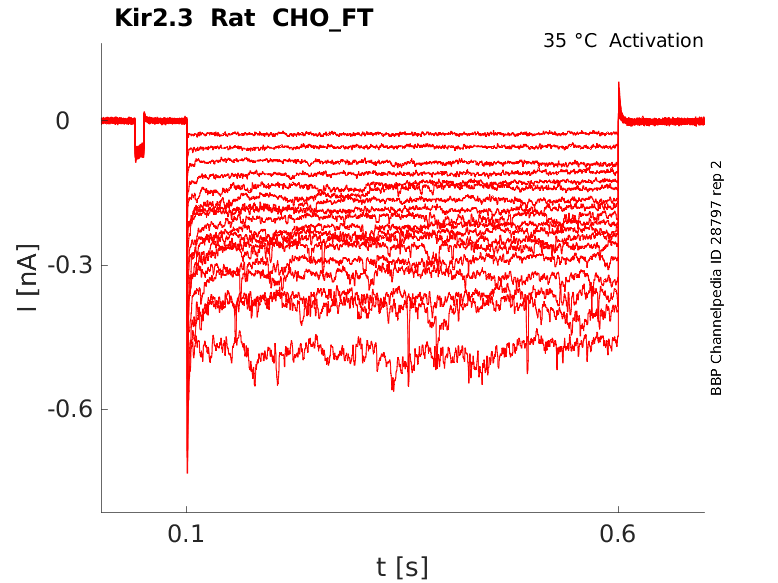
35 °Cshow 22 cells |
|
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_152868.3 | 2065 | |
| Mouse | NM_008427.5 | 2152 | |
| Rat | NM_053870.3 | 2656 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Structure
Kir2.3 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Human Kir2.3 in CHO cells Kinetics

Single Channel Conductance of Kir2.3
Expression of Kir2.3 in Mouse Brain
Although Kir2.3 is known to be expressed abundantly in the forebrain, its precise localization has not been identified. Using an antibody specific to Kir2.3, we examined the subcellular localization of Kir2.3 in mouse brain. Kir2.3 immunoreactivity was detected in a granular pattern in restricted areas of the brain, including the olfactory bulb (OB) [1879]
Expression of Kir2.3 in Rat Brain
we have provided both immunohisto- chemical and electrophysiological evidence that the Kir2.3 strong inward rectifier channel is expressed in astrocytes of both polygonal and stellate morphology, from both adult and neonatal rat brain, both in vivo and in vitro. Given the importance of this channel in maintaining the resting potential of polygonal reactive astrocytes [1880]
Expression of Kir2.3 in Mammalian CNS
Kir2.3 has previously been identified in mammalian CNS forebrain (Lesage et al., 1994; Morishige et al., 1994; Bredt et al., 1995; Karschin et al., 1996; Stone- house et al., 1999), in nodes of Ranvier (Mi et al., 1996), and in renal epithelial cells (Welling, 1997) [1880]
Kir2.3 is highly expressed in the heart and brain (Perier 1994 [14).
Subcellular Localization of Kir2.3
Immunoelectron microscopy of the OB revealed that Kir2.3 immunoreactivity was specifically clustered on the postsynaptic membrane of asymmetric synapses between granule cells and mitral/tufted cells [1879]
Kirs2.3 function in Rat Cardiomyocytes
A major finding of this study is that Kir2.3 channel subunit is essential for normal native IK1 currents in the neonatal rat cardiomyocytes. Previous studies showed that the deletion of Kir2.1 subunit almost eliminated the IK1 currents, and the deletion of Kir2.2 subunit reduced the IK1 currents by 50% [15]. Our data demonstrated that IK1 current densities in the Kir2.3 knock-down cardiomyocytes were decreased by about 80%. Therefore, Kir2.3 subunit also plays an important role in IK1 currents.
IN cardiac myocytes the inward rectifier potassium current, IK1, regulates the late phase of action potential (AP) repolarization and stabilizes the resting membrane potential. IK1 channels are believed to be homo- and/or heterotetramers of Kir2.1, Kir2.2, and Kir2.3 subunits from the Kir2 family of inward rectifier potassium channels (Lopatin [926], Nichols [928]). A number of studies consistently indicated that the species-dependent (Dhamoon [920]) and tissue-specific expression (Schram [929]) of different Kir2 subunits may contribute, at least in part, to its variability. There is evidence that, in mouse ventricles, Kir2.1 is the major isoform with a significant contribution of Kir2.2, although knockout of both genes revealed the presence of another slowly activating component characteristic of Kir2.3 subunits (Zaritzki [910]).
Temporal Lobe Epilepsy
Kir2.3, has been found down-regulated in the hippocampus of chronic temporal lobe epileptic (cTLE) patients which may underline the mechanism of epilepsy. Our results suggest that the down-regulation of the Kir2.3 channel expression might contribute to the pathogenesis of TLE, which may be ameliorated by the administration of tenidap [1881]
Free intracellular polyamines (PAs) are the key determinants of rectification in IK1 and Kir2 channels (Lopatin [931]). Yan et al. [930] provided evidence that different concentrations of free PAs may underlie differences between atrial and ventricular IK1 in the guinea pig heart.
Inclusion of only one Kir2.3 subunit to a Kir2.1 channel led to an approximate threefold slowing of activation kinetics, with greater slowing on subsequent additions of Kir2.3 subunits. Activation kinetics of IK1 in both ventricles and both atria was found to correspond to fast-activating Kir2.1/Kir2.2 channels, suggesting no major contribution of Kir2.3 subunits. Panama [183]
Kir2.3 is modulated by ATP (Collins et al., 1996 [933]), protein kinase C (PKC) (Henry et al., 1996 [934]), G protein beta-gamma subunits (Cohen et al., 1996 [935]), Mg2+ (Chuang et al., 1997 [936]), pH (Coulter et al., 1995 [937]; Zhu et al., 1999a [938]), arachidonic acid (AA) (Liu et al., 2001 [188]) and the membrane phospholipid phosphatidyl inositol 4,5-bisphosphate (PIP2) (Du et al., 2004 [939]). Listed by Wang [188].
References
Panama BK
et al.
Heterogeneity of IK1 in the mouse heart.
Am. J. Physiol. Heart Circ. Physiol.,
2007
Dec
, 293 (H3558-67).
Wang C
et al.
Arachidonic acid activates Kir2.3 channels by enhancing channel-phosphatidyl-inositol 4,5-bisphosphate interactions.
Mol. Pharmacol.,
2008
Apr
, 73 (1185-94).
Shyng SL
et al.
Depletion of intracellular polyamines relieves inward rectification of potassium channels.
Proc. Natl. Acad. Sci. U.S.A.,
1996
Oct
15
, 93 (12014-9).
Liu Y
et al.
Direct activation of an inwardly rectifying potassium channel by arachidonic acid.
Mol. Pharmacol.,
2001
May
, 59 (1061-8).
Dyachenko V
et al.
Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels.
Cell Calcium,
2009
Jan
, 45 (38-54).
Tikku S
et al.
Relationship between Kir2.1/Kir2.3 activity and their distributions between cholesterol-rich and cholesterol-poor membrane domains.
Am. J. Physiol., Cell Physiol.,
2007
Jul
, 293 (C440-50).
Zaritsky JJ
et al.
The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes.
J. Physiol. (Lond.),
2001
Jun
15
, 533 (697-710).
Dhamoon AS
et al.
Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current.
Circ. Res.,
2004
May
28
, 94 (1332-9).
Lopatin AN
et al.
Inward rectifiers in the heart: an update on I(K1).
J. Mol. Cell. Cardiol.,
2001
Apr
, 33 (625-38).
Schram G
et al.
Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function.
Circ. Res.,
2002
May
17
, 90 (939-50).
Yan DH
et al.
Different intracellular polyamine concentrations underlie the difference in the inward rectifier K(+) currents in atria and ventricles of the guinea-pig heart.
J. Physiol. (Lond.),
2005
Mar
15
, 563 (713-24).
Lopatin AN
et al.
The mechanism of inward rectification of potassium channels: "long-pore plugging" by cytoplasmic polyamines.
J. Gen. Physiol.,
1995
Nov
, 106 (923-55).
Périer F
et al.
Primary structure and characterization of a small-conductance inwardly rectifying potassium channel from human hippocampus.
Proc. Natl. Acad. Sci. U.S.A.,
1994
Jun
21
, 91 (6240-4).
Collins A
et al.
A strongly inwardly rectifying K+ channel that is sensitive to ATP.
J. Neurosci.,
1996
Jan
, 16 (1-9).
Henry P
et al.
Protein kinase C inhibition of cloned inward rectifier (HRK1/KIR2.3) K+ channels expressed in Xenopus oocytes.
J. Physiol. (Lond.),
1996
Sep
15
, 495 ( Pt 3) (681-8).
Cohen NA
et al.
Inhibition of an inward rectifier potassium channel (Kir2.3) by G-protein betagamma subunits.
J. Biol. Chem.,
1996
Dec
13
, 271 (32301-5).
Chuang H
et al.
Regulation of IRK3 inward rectifier K+ channel by m1 acetylcholine receptor and intracellular magnesium.
Cell,
1997
Jun
27
, 89 (1121-32).
Coulter KL
et al.
Identification and molecular localization of a pH-sensing domain for the inward rectifier potassium channel HIR.
Neuron,
1995
Nov
, 15 (1157-68).
Zhu G
et al.
Effects of intra- and extracellular acidifications on single channel Kir2.3 currents.
J. Physiol. (Lond.),
1999
May
1
, 516 ( Pt 3) (699-710).
Du X
et al.
Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators.
J. Biol. Chem.,
2004
Sep
3
, 279 (37271-81).
Inanobe A
et al.
Inward rectifier K+ channel Kir2.3 is localized at the postsynaptic membrane of excitatory synapses.
Am. J. Physiol., Cell Physiol.,
2002
Jun
, 282 (C1396-403).
Perillán PR
et al.
Inward rectifier K(+) channel Kir2.3 (IRK3) in reactive astrocytes from adult rat brain.
Glia,
2000
Aug
, 31 (181-92).
Xu L
et al.
Tenidap, an agonist of the inwardly rectifying K+ channel Kir2.3, delays the onset of cortical epileptiform activity in a model of chronic temporal lobe epilepsy.
Neurol. Res.,
2013
Jul
, 35 (561-7).
Credits
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/44/ , accessed on 2026 Feb 19
