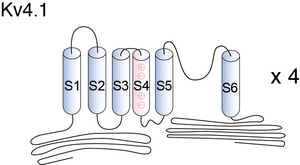
Kv4.1
Description: potassium voltage-gated channel, Shal-related subfamily, member 1 Gene: Kcnd1 Alias: Kv4.1, kcnd1, Kca2-1, mShal1, Shal
Kv4.1, encoded by the gene KCND1, is a member of the potassium voltage-gated channel subfamily D. Kv4.1 plays a role in the repolarization phase of the action potential. Kv4.1 is expressed at moderate levels in all tissues analyzed, with lower levels in skeletal muscle.NCBI
Experimental data
Rat Kv4.1 gene in CHO host cells datasheet |
||
|
Click for details 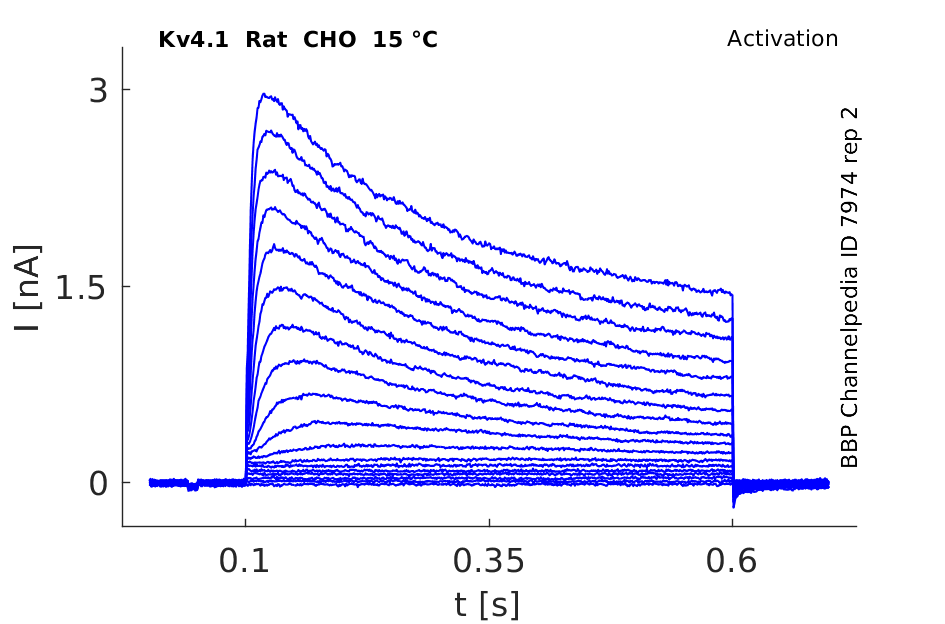
15 °Cshow 62 cells |
Click for details 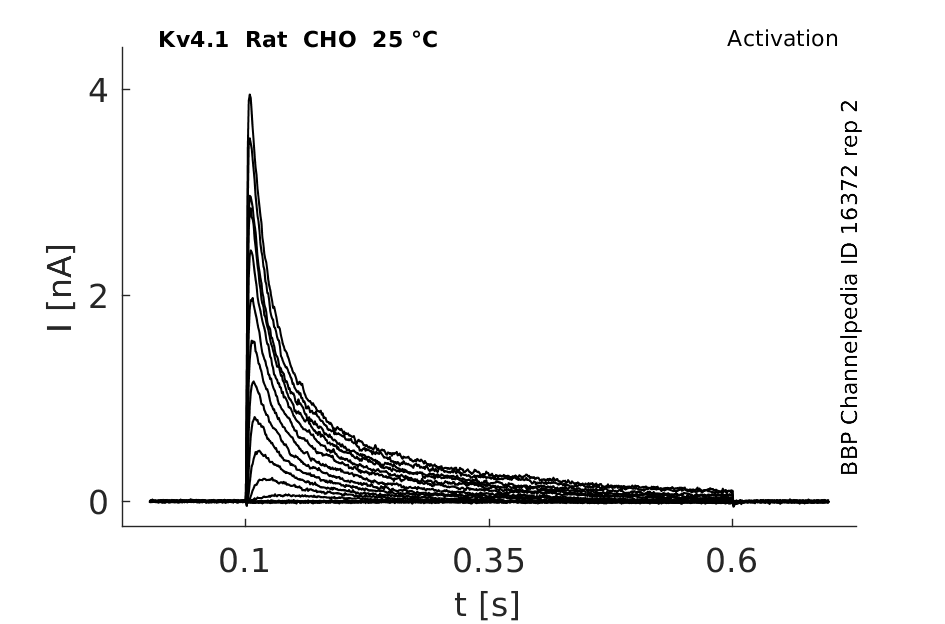
25 °Cshow 125 cells |
Click for details 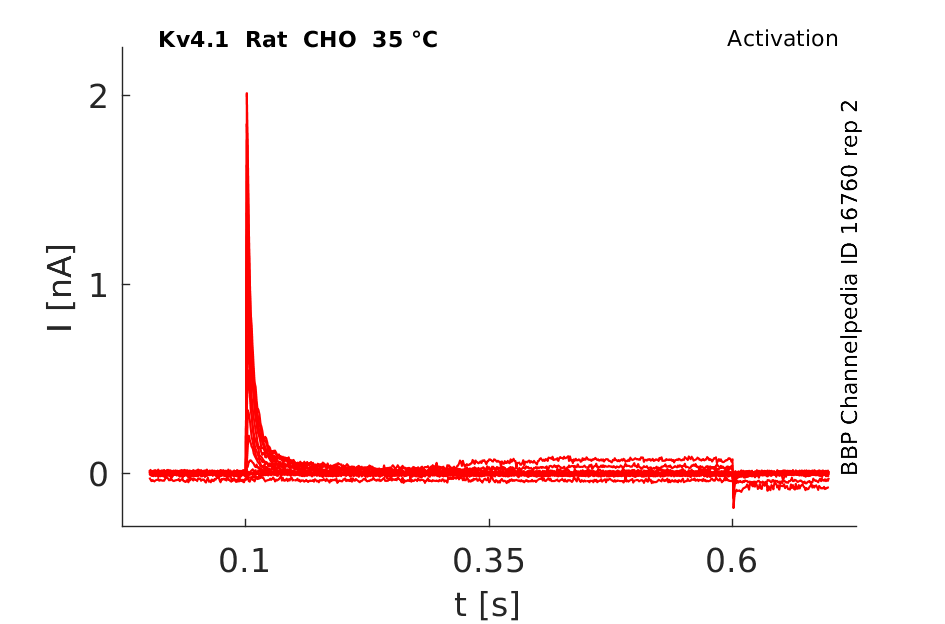
35 °Cshow 230 cells |
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_004979.6 | 4718 | |
| Mouse | NM_008423.2 | 3815 | |
| Rat | NM_001105748.1 | 3712 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv4.1 Structure
Methodology for visual representation of structure available here
Stereo view of binding site in T1 domain of Kv4.1

Kv Proteins have a core membrane consisting of six putative membrane- spanning domains designated S1, S2, S3, S4, S5, and S6 flanked by intracellular amino and carboxyl domainsof vari- able lengths, and an H5 or pore (P) domain (between the S5 and S6 membranespanning sequences)thought to contribute to the channel’s pore. The amphipatic S4 domain is believed to be a key part of the voltage sensor and has a number of positively charged residuesthat is characteristic for each subfamily of proteins (5 in Shal subunits) [395]
Kv4.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Single Channel Conductance

Channel Kinetics with and without KChip1

Although Kv4.1 and Kv4.2 share high sequence similarity, KChIP1 has opposite effects on their voltage dependence of activation and the inactivation time courses. KChIP1 slows Kv4.2 inactivation but accelerates the Kv4.1 inactivation time course [1563]
Homomeric channels arising from Kv1.4, Kv3.4, and Kv4.1, Kv4.2, and Kv4.3 subunits give rise to A-type channels [274], [636], [635], [395].
Kinetic Model
The two main kinetic features of Model 1 are, first, that Kv4.1 channels have to return from the short-lived open-inactivated state IO via the open state to the pre-open closed state before they can enter the closed-inactivated state, IC, and second, that cumulative Kv4.1 channel inactivation is a two-step reaction involving the closed-inactivated state, IC, and the deep-inactivated state, ID [30]
Markov Model Kv4.1
Kv channels have been considered important in excitable cells such as neurons and myocytes where they are involved in the regulation of membrane potential, and the generation and propagation of action potentials. [637], [638], [639], [640], [556]. However, several subtypes of Kv channels are also expressed in non-excitable cells such as epithelial cells, where they contribute to cell migration and wound healing, O2 sensing, cell proliferation, and apoptosis.[641], [642], [643], [644].
Kv4.1, one member of the Kv channel family, was originally cloned from brain tissue, [645] where it exists at a low level [395]. According to several recent reports, Kv4.1 mRNA and protein have been detected in epithelial cells, including alveolar and mammary epithelial cells and adipose tissue-derived stem cells. [397], [646] ,[647].
Kv4.1 mRNA-positive cells represented 59.5 % of DRG (dorsal root ganglion) neurons [1561]
In this study, we found that Kv4.1 and Kv4.2 channel subunits of the Shal-related family were expressed in the SCN (suprachiasmatic nucleus) [1566]
These results argue that the depolarization-activated somatodendritic K+ currents in cholinergic interneurons are dominated by Kv4.2- and Kv4.1-containing channels [26]
Striatal cholinergic interneurons coexpress several of the alpha and beta subunits known to produce A-type channels but, within the somatodendritic membrane, Kv4.2 and Kv4.1 channels are the predominate channel types and possess the biophysical properties necessary to regulate repetitive discharge. [26]
CANCERS
Kv4.1 expression was proven in breast cancer cells and it is thought to play a role in cell proliferation.[397]
Kv4.1 mRNA and protein are expressed in the human gastric cancer cell lines MKN-45 and SNU-638 and breast cancer cells [397]
Silencing of Kv4.1 expression with siRNA-Kv4.1 inhibited cell proliferation of tumorigenic M13SVR2 and M13SV1R2-N1 cells, but not immortal M13SV1 cells. Although the involvement of Kv4.1 channel subtypes in tumor cell proliferation has not been extensively investigated, a recent study demonstrated that Kv4 may play a role in tumorigenesis of pituitary adenomas [643]
Oxygen regulators
Furthermore, several lines of evidence support the conclusion that Kv4 channel subfamily members (either Kv4.3 alone or Kv4.3/Kv4.1 heteromultimers) are the oxygen sensitive K channels (K(o2)) in rabbit CB chemoreceptor cells [1567]
Regulatory Volume Decrease
Kv4.1 and (or) Kv4.3 play a crucial role in mediating this RVD response [1568]
In the nervous system, Kv4 channels prevent backpropagating action potentials, help to establish slow repetitive spike firing and contribute to spike repolarization and signal amplification [25]
Alzheimers Disease
Changes in Kv4.x channels may also occur in Alzheimer's disease. Mutations in presenilins have been linked to early-onset, autosomal dominant familial Alzheimer's disease [466]
KChIP1
Caused a small but significant depolarizing shift in Kv4.1 activation and KChIP1 markedly accelerated Kv4.1 inactivation. [1563]
Frequenin
Frequenin had relatively modest effects on Kv4.1 currents. Although in some batches of oocytes frequenin increased Kv4.1 current amplitudes, the overall effect was not statistically significant and the inactivation kinetics were largely unaffected. Curve fitting of Kv4.1 current traces to a sum of three exponentials showed that frequenin affected neither the time constants nor the relative contribution of inactivation components [1564]
Jingzhaotoxin-XII
Jingzhaotoxin-XII (JZTX-XII), a 29-residue polypeptide, was purified from the venom of the Chinese tarantula Chilobrachys jingzhao. Electrophysiological recordings carried out in Xenopus laevis oocytes showed that JZTX-XII is specific for Kv4.1 channels, with the IC50 value of 0.363 μM
Heteropoda toxin 2
(HpTx2) is an inhibitor cysteine knot peptide toxin specific for Kv4 channels. HpTx2 interaction with Kv4.1 is considerably less voltage-dependent, has smaller shifts in the voltage-dependences of conductance and steady-state inactivation, and a 3-fold higher K(d) value than Kv4.3 [1565]
DPPX
Here, we explore the presence and functional contribution of DPPX to K(o2) currents (4.1/4.3) in rabbit CB chemoreceptor cells by using DPPX functional knockdown with siRNA. Our data suggest that DPPX proteins are integral components of K(o2) currents, and that their association with Kv4.1 subunits modulate the pharmacological profile of the heteromultimers [1567]
Estrogen
We found that EB significantly increased the expression of Kv4.1 in the rostral arcuate. In the caudal arcuate, EB also increased the expression of Kv4.1 [1569]
sBmTX3
The rapidly activating and inactivating Kv4.1 current was inhibited by sBmTX3, a chemically synthesized toxin originally purified from the venom of the scorpion Buthus martensi (IC50, 105 nM) [1570]
Arachidonic Acid
Arachidonic acid (20uM) selectively inhibits Kv4.1 by almost 50% [1572]
References
Beck EJ
et al.
Remodelling inactivation gating of Kv4 channels by KChIP1, a small-molecular-weight calcium-binding protein.
J. Physiol. (Lond.),
2002
Feb
1
, 538 (691-706).
Song WJ
et al.
Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits.
J. Neurosci.,
1998
May
1
, 18 (3124-37).
Bähring R
et al.
Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels.
J. Physiol. (Lond.),
2001
Aug
15
, 535 (65-81).
Stühmer W
et al.
Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain.
EMBO J.,
1989
Nov
, 8 (3235-44).
Serôdio P
et al.
Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain.
J. Neurophysiol.,
1996
May
, 75 (2174-9).
Isbrandt D
et al.
Gene structures and expression profiles of three human KCND (Kv4) potassium channels mediating A-type currents I(TO) and I(SA).
Genomics,
2000
Mar
1
, 64 (144-54).
Kim HJ
et al.
Involvement of Kv4.1 K(+) channels in gastric cancer cell proliferation.
Biol. Pharm. Bull.,
2010
, 33 (1754-7).
Birnbaum SG
et al.
Structure and function of Kv4-family transient potassium channels.
Physiol. Rev.,
2004
Jul
, 84 (803-33).
Schröter KH
et al.
Cloning and functional expression of a TEA-sensitive A-type potassium channel from rat brain.
FEBS Lett.,
1991
Jan
28
, 278 (211-6).
Baldwin TJ
et al.
Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel.
Neuron,
1991
Sep
, 7 (471-83).
Jan LY
et al.
Voltage-gated and inwardly rectifying potassium channels.
J. Physiol. (Lond.),
1997
Dec
1
, 505 ( Pt 2) (267-82).
Pichon Y
et al.
Some aspects of the physiological role of ion channels in the nervous system.
Eur. Biophys. J.,
2004
May
, 33 (211-26).
Yellen G
The voltage-gated potassium channels and their relatives.
Nature,
2002
Sep
5
, 419 (35-42).
O'Grady SM
et al.
Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells.
Int. J. Biochem. Cell Biol.,
2005
Aug
, 37 (1578-94).
Lotz MM
et al.
K+ channel inhibition accelerates intestinal epithelial cell wound healing.
,
2004 Sep-Oct
, 12 (565-74).
Jang SH
et al.
Silencing of Kv4.1 potassium channels inhibits cell proliferation of tumorigenic human mammary epithelial cells.
Biochem. Biophys. Res. Commun.,
2009
Jun
26
, 384 (180-6).
Jang SH
et al.
Kv1.3 voltage-gated K(+) channel subunit as a potential diagnostic marker and therapeutic target for breast cancer.
BMB Rep,
2009
Aug
31
, 42 (535-9).
Pak MD
et al.
mShal, a subfamily of A-type K+ channel cloned from mammalian brain.
Proc. Natl. Acad. Sci. U.S.A.,
1991
May
15
, 88 (4386-90).
Lee SY
et al.
Adult alveolar epithelial cells express multiple subtypes of voltage-gated K+ channels that are located in apical membrane.
Am. J. Physiol., Cell Physiol.,
2003
Jun
, 284 (C1614-24).
Bai X
et al.
Electrophysiological properties of human adipose tissue-derived stem cells.
Am. J. Physiol., Cell Physiol.,
2007
Nov
, 293 (C1539-50).
Matsuyoshi H
et al.
Distinct cellular distributions of Kv4 pore-forming and auxiliary subunits in rat dorsal root ganglion neurons.
Life Sci.,
2012
Sep
17
, 91 (258-63).
Covarrubias M
et al.
The neuronal Kv4 channel complex.
Neurochem. Res.,
2008
Aug
, 33 (1558-67).
Nakamura TY
et al.
Different effects of the Ca(2+)-binding protein, KChIP1, on two Kv4 subfamily members, Kv4.1 and Kv4.2.
FEBS Lett.,
2001
Jun
22
, 499 (205-9).
Nakamura TY
et al.
A role for frequenin, a Ca2+-binding protein, as a regulator of Kv4 K+-currents.
Proc. Natl. Acad. Sci. U.S.A.,
2001
Oct
23
, 98 (12808-13).
DeSimone CV
et al.
Heteropoda toxin 2 interaction with Kv4.3 and Kv4.1 reveals differences in gating modification.
,
2011
May
3
, ().
Itri JN
et al.
Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus.
J. Neurophysiol.,
2010
Feb
, 103 (632-40).
Colinas O
et al.
DPPX modifies TEA sensitivity of the Kv4 channels in rabbit carotid body chemoreceptor cells.
Adv. Exp. Med. Biol.,
2009
, 648 (73-82).
Harron SA
et al.
Volume regulation in the human airway epithelial cell line Calu-3.
Can. J. Physiol. Pharmacol.,
2009
May
, 87 (337-46).
Roepke TA
et al.
Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus.
Endocrinology,
2007
Oct
, 148 (4937-51).
Vacher H
et al.
Kv4 channels sensitive to BmTX3 in rat nervous system: autoradiographic analysis of their distribution during brain ontogenesis.
Eur. J. Neurosci.,
2006
Sep
, 24 (1325-40).
Wang G
et al.
Functionally active t1-t1 interfaces revealed by the accessibility of intracellular thiolate groups in kv4 channels.
J. Gen. Physiol.,
2005
Jul
, 126 (55-69).
Villarroel A
et al.
Inhibition of the Kv4 (Shal) family of transient K+ currents by arachidonic acid.
J. Neurosci.,
1996
Apr
15
, 16 (2522-32).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/15/ , accessed on 2026 Feb 27