Kv1.3
Description: potassium voltage-gated channel, shaker-related subfamily, member 3 Gene: Kcna3 Alias: Kv1.3, kcna3
Kv1.3 (also known as MK3; HGK5; HLK3; PCN3; HPCN3; KV1.3; HUKIII), encoded by the gene KCNA3, is a member of the potassium voltage-gated channel subfamily A. Kv1.2 plays an essential role in T cell proliferation and activation. Kv1.3 channels are expressed in in various tissues and cell types including brain, vascular smooth muscle cells and leucocytes. Malfunction of Kv1.3 can alter the immune response, elicit neurotoxic effects or impact on cancer growth.[2002][1525]
Experimental data
Rat Kv1.3 gene in CHO host cells datasheet |
||
|
Click for details 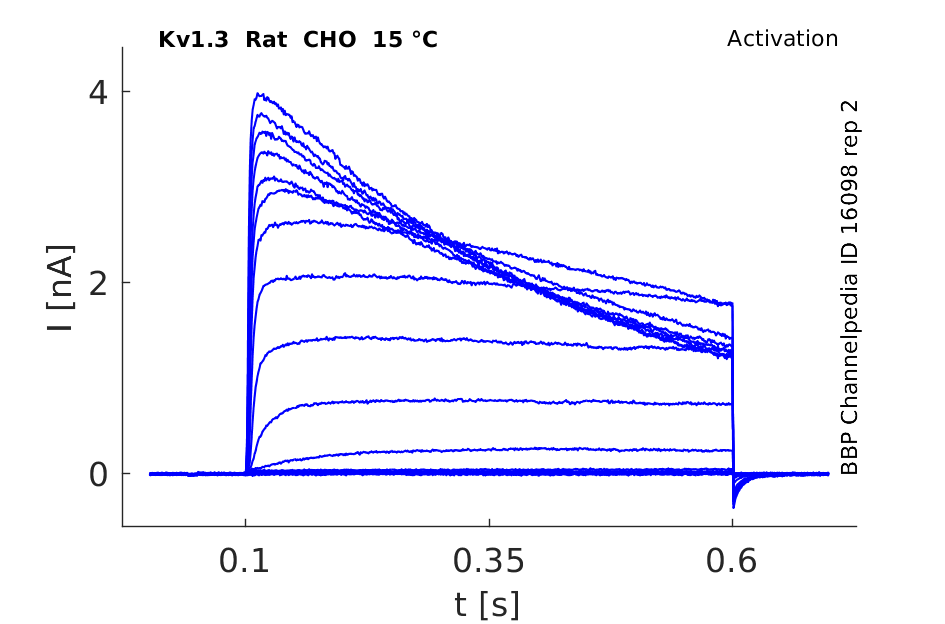
15 °Cshow 103 cells |
Click for details 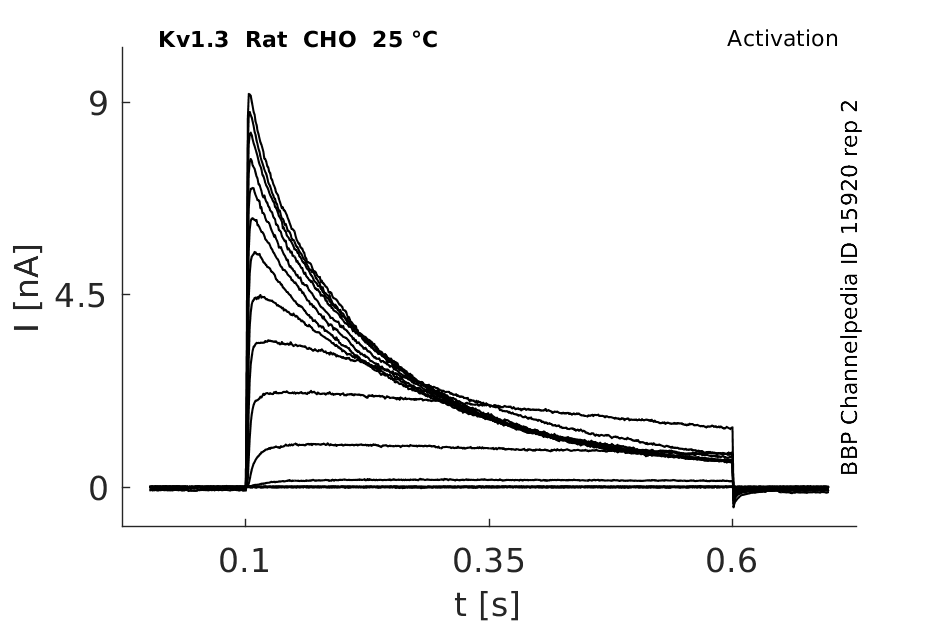
25 °Cshow 231 cells |
Click for details 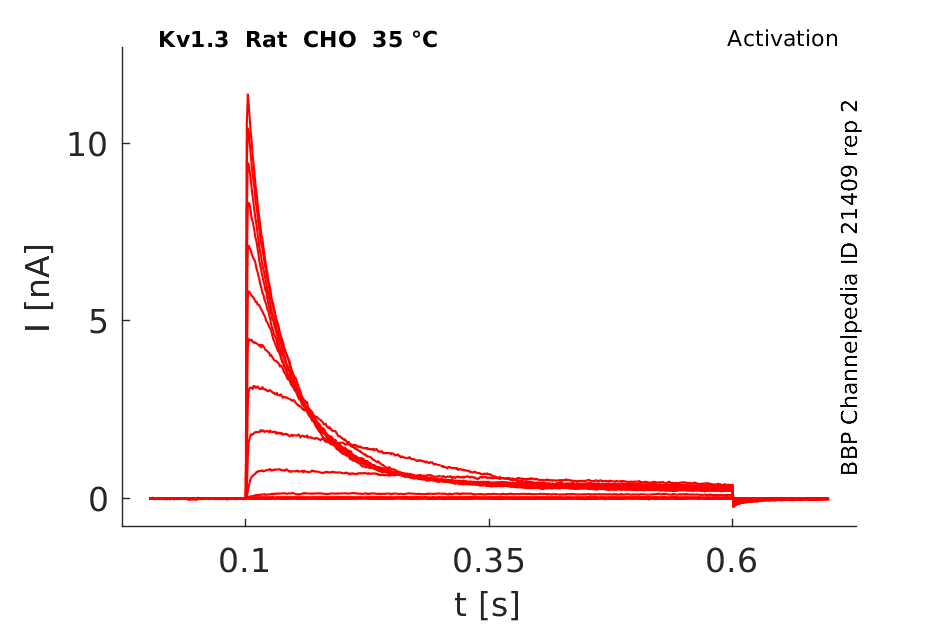
35 °Cshow 179 cells |
This gene appears to be intronless and is clustered together with KCNA2 and KCNA10 genes on chromosome 1.
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_002232.5 | 2476 | |
| Mouse | NM_008418.2 | 1968 | |
| Rat | NM_019270.3 | 1578 |
Protein Isoforms
Isoforms
Post-Translational Modifications
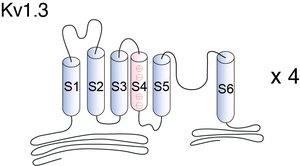
Visual Representation of Kv1.3 Structure
Methodology for visual representation of structure available here
Voltage-gated K+ channels (Kv) are tetrameric molecules with six putative a-helical transmembrane segments (TM) linked by extra- and intracellular loops: the fifth and the sixth (S5-S6) TM’s and the connecting extracellular loop line the pore region and make up the conducting pore, whereas the first four (S1-S4) TM’s are thought to scaffold the activation gate of these channels [580].
High Resolution Crystallography
Using a high resolution crystal structure of Kv1.3, we see that Kv1.3-T1(51–155) is very similar to the known structures of the T1 domains derived from Aplysia Kv1.1 and rat Kv1.2. Ribbon-type representations of the tetramer are shown in Fig. 1a, b. The monomer consists of an N-terminal b-strand rich domain and a C-terminal a- helical domain [1538]
The most remarkable difference to the already published T1 domain structures is the occurrence of more than one side chain conformation for the residues Val55, Ile56, Leu77, Arg86, Tyr109, Arg116, Ser129, Glu140, Met142, and Arg146 [1538]
Another difference to known structures is the slight but significant rotational displacement of the individual monomers in the tetrameric arrangement of the human Kv1.3 T1 domain. Such a rotational rearrangement in the T1 domain has been hypothesized to explain voltage- dependent gating rearrangement in the intracellular T1–T1 interfaces of Kv4 potassium channels observed through cysteine accessibility studies [1538]
Kv1.3 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
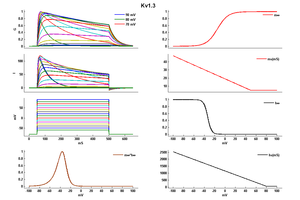
Kinetics
Kv channels open on membrane depolarization, then get into a non-conducting, inactivated state. The activation of Kv channels is thought to be a conformational change initiated in the first four segments of the channel protein, while inactivation can occur by two mechanisms, called N-type and C-type [[581]] (#a581).
Single Channel Current
The single channel conductance of Kv1.3 is 13 pS, and the voltage required for activation is −35 mV [1525]
PK antagonists speed up inactivation of Kv1.3 current
To clarify the role of protein kinases in the regulation of Kv1.3 channels segregated into the IS we applied H89, GF109203X (GF), and damnacanthal (DAM), inhibitors of PKA (Kd ≈ 50 nM), PKC (Kd ≈ 14 nM), and p56lck (Kd = 17 and 620 nM), respectively. It was reported previously that these compounds have direct blocking effect on ion channels: H89 blocks Kv1.3 with an IC50 of 1.7 μM [1528]
Kv1.3 Kinetics Graph
Kv1.3 currents (black) exhibited a fast activation and then a slow inactivation, evoked by 1 s depolarizing voltage steps ranging from −70 to +50 mV in 15 mV increments from a 90 s holding potential of −90 mV to remove the possible inactivation. [1532]
Kv1.3 Kinetics in CHO cells

Kv1.3 currents recorded in Xenopus oocytes modulation by VP
Tracings were recorded in Xenopus oocytes injected with cRNA encoding Kv1.3 after 2 s of depolarizing pulses of -80 to +50 mV in 10 mV steps at a holding potential of -100mV. The coexpression of parvovirus B19 capsid protein VP1 significantly decreased K+ currents.[1988]
Kv1.3 steady-state activation in HEK 293T cells and blocking by Ctri9577
The steady-state activation of Kv1.3 channels was measured with a one-pulse protocol in HEK 293 cells. 200 ms voltage pulses were applied from -80 mV to 0 mV in 5 mV steps using a holding potential of -80 mV. The blocking process of the scorpion toxin Ctri9577 was shown not to be voltage -dependend for Kv1.3 channels.[1998]
Kv1.3 currents measured in Xenopus oocytes and enhancment by Klotho
K+ currents were measured in Xenopus oocytes injected with cRNA for KCNA3. Human Klotho protein significantly enhanced the Kv1.3 currents as well as the abundance of Kv1.3 expression.[1997]
Kinetic Model
A simple sequential model for simultaneously simulating the whole kinetics of Kv1.3 currents including activation, deactivation, steady-state inactivation and recovery
HODGKIN/HUXLEY MODEL

Model Kv1.3 (ID=38)
| Animal | rat | |
| CellType | Oocyte | |
| Age | 0 Days | |
| Temperature | 0.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [273] J P Adelman et. al; J. Immunol. 1990 Jun 15 | |
| mpower | 1.0 | |
| m Inf | 1.0000/(1+ exp((v - -14.1000)/-10.3000)) | |
| m Tau | (-0.2840 * v) + 19.1600 If v lt 50 | |
| m Tau | 5 If v gteq 50 | |
| hpower | 1.0 | |
| h Inf | 1.0000/(1+ exp((v - -33.0000)/3.7000)) | |
| h Tau | (-13.7600 * v) + 1162.4000 If v lt 80 | |
| h Tau | 60 If v gteq 80 | |
In Rhesus Macaque T-cells, the Kv1.3 expression pattern is similar to that in human T-cells [116].
While Kv1.3 channels are found in all T and B cell subsets in the resting state, their expression is markedly upregulated in activated effector memory T cells (TEMs) and Ig class-switched memory B cells from ~250 to ~1500 channels per cell [577],[578].
Kv1.3 immunoreactivity was found in the cerebellum, olfactory bulb, hippocampus, and cerebral cortex, particularly in frontal cortex. In coronal sections of the brainstem, prominent Kv1.3 immunoreactivity was found bilaterally in the MNTB, a nucleus that plays a key role in circuits that detect the localization of sounds in the azimuth. Lower levels of Kv1.3 were also detected in several other auditory brainstem nuclei [374]
Kv1.3 is also present in the central nervous system, kidney, liver, skeletal muscle, platelets, macrophages, testis, and osteoclasts [1530]
DENDRITIC CELLS
Potassium Channels Kv1.3 and Kv1.5 Are Expressed on Blood-Derived Dendritic Cells in the Central Nervous System [1703]
LYMPHOCYTES
To determine the cellular localization of Kv1.3, transmission electron microscopy studies were performed on freshly isolated peripheral human blood lymphocytes. These experiments surprisingly demonstrated the presence of the channel protein in mitochondria and confirmed the previously defined localization of Kv1.3 in the plasma membrane and in intracellular vesicles, most likely of the endoplasmic reticulum [1533]
Mitral cells
Kv1.3 expression in mitral cells of the olfactory bulb was shown to be involved in the mediation of glucose-sensitivity in these projection neurons [1944]
Nuclear distribution in cancer and neural tissue
Kv1.3 channels are primarily localized in the nucleus of several types of cells in cancer and in brain tissue. This nuclear distribution of Kv1.3 may be associated with gene regulation activity of Sp1[1980]
Regulates membrane potential in T cells
Kv1.3 regulates membrane potential and Ca2+ signaling in human T cells, and its expression is increased 4- to 5-fold in activated CD4+ and CD8+ TEM/TEMRA cells [1530]
Regulates physiological functions
Voltage-gated potassium channels (Kv) regulate several physiological functions of lymphocytes including membrane potassium permeability, calcium influx, cytokine production, clonal expansion and cell death [573], [574], [575], [576].
Acute Coronary Syndrome
Majority of patients with acute coronary syndrome have increased Kv1.3 activity in CD4+CD28null cells in peripheral blood and that the pro-inflammatory and cytotoxic activities of these cells can be reduced by Kv1.3 inhibitors [1529]
Autoimmune Disorders
The two major K+ channels that are expressed in lymphocytes, Kv1.3 and KCa3.1, are promising targets for the treatment of autoimmune disorders. Previous studies have described protective effects of Kv1.3 inhibitors or gene deletion on multiple sclerosis, type 1 diabetes, rheumatoid arthritis, psoriasis, and rapidly progressive glomerulonephritis. Moreover, KCa3.1 is related to acute immune responses and Kv1.3 is related to chronic immune responses, the combined administration with Kv1.3 and KCa3.1 inhibitors is likely to enhance their effects in autoimmune disorders or graft rejection [1700]
Cancers
The expression and relationship of Kv1.3 ion channel on several types of cancers (breast, prostate, muscle sarcoma, gliomas, leukemias and blood cancers) has been studied substantially [1525] Expression of Kv1.3 channels in colorectal cancers may point at an involvment of this channel-type in their formation [1986] Kv1.3channels also exhibited high expression levels in human osteosarcoma and were shown to promote their growth. This may indicate Kv1.3 channels as putative therapeutic targets [2004] Expression of Kv1.1 and Kv1.3 was shown to sensitize tumour cells of various origins to cytotoxins [1899] KCNA3 was reported as an efficient marker gene for parathyroid carcinoma [2006]
Integrin Signalling
In nonneuronal cells, the Kv1.3 channel forms a tight physical link with b-integrins, and the activity of the channel has been found to regulate integrin signaling and cell proliferation as well as the killing of neurons by microglia [374]
Diabetes
Kv1.3 inhibitors ameliorate pristane-induced arthritis in rats and reduce the incidence of experimental autoimmune diabetes in diabetes-prone (DP-BB/W) rats [1530]
Cell Death
Examination of Kv1.3-deficient mice led to the discovery that Kv1.3, which seems to be abundantly expressed in brown and white fat, regulates energy homeostasis and body weight. Kv1.3 might therefore exert its regulatory effect on the basal metabolic rate directly in the mitochondria. Further finding that mitochondria contain Kv1.3 may also provide a plausible explanation for the fact that in actinomycin D-induced cell death mitochondria-associated events required expression of Kv1.3 [1533]
MS
The expression pattern revealed not only Kv1.3+ T cells in the perivenular infiltrate but also high expression in the parenchyma of demyelinated MS lesions and both normal appearing gray and white matter. This provides further rationale for the use of specific Kv1.3 antagonists in MS [1533]
Regulates Insulin
Kv1.3 also found to regulate Insulin in the periphery [1538]
Inflammation-mediated neurological disorders
Kv1.3 is involved microglia-mediated neurotoxic activity in HIV-1-infected brains, pointing at Kv1.3 inhibitors as novel therapeutics for inflammation-mediated neurological disorders [1945]
Regulation of cortical interneuron density
Expression of Kv1.3 channels is linked to the density of cortical interneuron types. KCNA null mice displayed an increase in the number of parvalbumin (PV) cells and a decrease in the number of calbindin (CB), calretinin (CR), neuropeptide Y (NPY), vasoactive intestinal peptide (VIP), and somatostatin (SOM)expressing interneurons, as well as an overall reduction of cortical thickness [1946]
Kv1.3 and leukocyte activity
Kv1.3 channels were shown to have a modulatory effect on the cholesterol metabolism in macrophages. Specific Kv1.3 blockade may therefore constitute a novel strategy of therapeutic intervention in this metabolic process [1952] Kv1.3 blockade was also shown to enhance the phagocytic function in RAW264.7 macrophages [1971]
Blockade of Kv1.3 inhibited CD8+ T cell proliferation, secretion of GrB, and ability to kill neuron progenitors [1954] Proinflammatory cytokine secretion was also reduced in T(EM) cells by inhibition of hKv1.3 with curcumin [1953] Accordingly, B lymphocyte proliferation and Ly-6Chi monocyte chemotaxis were promoted by the opening of KCa3.1 and Kv1.3 channels [1974]Also PSD-95 targeting of Kv1.3 to the immunological synapse, during T-cell activation, implies a role of Kv1.3 channels in the activation process [1958] The effects of Kv1.3 inhibition on leukocytes make Kv1.3 a potential target for immunosuppression during allotransplantation [1956]
Kv1.3 in Immune-mediated disorders
Kv1.3 blockers ameliorate allergic contact dermatitis by suppressing effector memory T cells in a rat model [1960] Blocking KV1.3 channels was shown to inhibit Th2 lymphocyte function and to treat a rat model of asthma [1526] KV1.3 expression in T cells correlated with pro-inflammatory cytokines in ulcerative colitis [1963] Kv1.3 inhibition in lymphocytes was shown to have a therapeutic potential in acute ischemic stroke [1965] The effectivness of Kv1.3 inhibition in treating psoriatic disease was demostrated by PAP-1 inhibition of Kv1.3 in the mouse model [1966] Functional evidence has been provided for the role of Kv1.3 channels in lymphocytes of spontaneously hypertensive rats during aging [1970] Also, in patients with hypertension an increase of Kv1.3 and KCa3.1 expression in T cells has been reported [1973] Kv1.3 and IKCa1 channel inhibition alters calcium influx in human peripheral T lymphocytes in rheumatoid arthritis [1950]
Secretory pathway of Kv1.3
According to a mutation study the pore loop residues of Kv1.3 form a canonical α-helix within the monomer during the early biogenesis [1977] The posttranslational modification of Kv1.3 is localized to the cis-Golgi while that of Kv1.6 is localized to the endoplasmic reticulum in astrocytes. [1978] Kv1.3 contains an alternative ER exit motif involved in recruiting the channel into COPII vesicles by Sec24a [1981] A further, previously unrecognized motif of Kv channels is essential for their expression on the cell surface [1976] Membrane motility of Kv1.3 channels is then regulated by actin dynamics and mediated by the SH3 motif of Kv1.3 and cortactin [1979] Kv1.3 channels may undergo endocytosis initiated by EGF-dependent ERK1/2 activation and subsequent phosphorilation of threonine residues [1982]
Kv1.3-induced proliferation
Kv1.3-induced cell proliferation involves accessibility of C-terminus(Y447) and phosphorylation via MEK/ERK [1983]
Atherosclerosis
Changes in Kv1.3 channel gating behaviour in T lymphocytes have been associated with atherosclerosis [1999] Kv1.3 channels activity may also contribute to atherosclerosis by stimulating macrophage migration through the activation of ERK [2000] The importance of Kv1.3 in atherosclerosis is further supported by the finding that the Kv1.3 blocker PAP-1 can suppress the development of AS in a rat model [1529]
Neurotoxicity
Kaliotoxin (KTX) is a neurotoxin purified from the androctonus scorpion venom causing neuropathophysiological and immuno-inflammatory effects. Kv1.1 and Kv1.3 channels are targeted by KTX-binding in the CNS inducing severe modifications though the activation of immuno-inflammatory reactions [2001]Kv1.3 function was suggested to play a role in neurotoxic pathways. ShK-170 blockade of Kv1.3 ameliorates radiation-induced brain injury by suppression of microglial neurotoxicity [2002] Kv1.3 channel activity was also linked to methamphetamine-mediated microglial damage [2003]
Vascular smooth muscle cells
Kv1.3 channel blockers decrease proliferation, migration and hyperplasia of vascular smooth muscle cells (VSMCs), indicating a role of the channel in these processes. Kv1.3 channels were therefore suggested as as novel therapeutical targets in restenosis [2005] The involvement of Kv1.3 channels in the phenotypic modulation of human VSMCs highlights Kv1.3 as an alternative target to blocking mTOR [2007]
Neuron density
Deletion of the Kv1.3 alters mitral cell current properties contributing to a "Super-smeller" phenotype. An increase in mitral cell density was observed in these Kv1.3-null mice [2008]
Neuron differentiation and maturation
Kv1.3 channels may play a crucial role of in the regulation of neuroprogenitor cell differentiation and maturation. Blocking Kv1.3 with Psora-4 promoted neural differentiation and maturation [2010]
Pacemaking activity in SNc
Kv1.3 was identified as one of the subunits of voltage-gated ion channels in the substantia nigra pars compacta (SNc) that contribute to the somatodendritic delayed rectifier in dopaminergic neurons, others being Kv2.1, Kv3.2, Kv3.3, Kv4.3 [2009]
PAP-1
(5-(4-phenoxybutoxy)psoralen), PAP-1, is a synthetic derivate of the natural product 5-methoxypsoralen and exhibits a 23 to 125-fold selectivity for Kv1.3 over other members of the Kv1 family, effectively blocking Kv1.3 with an EC50 of 2 nM and a stoichiometry of 2 inhibitor molecules per channel [579].
MbCD and MbCD/C
Manipulation of membrane cholesterol oligosaccharide methyl-b-cyclodextrin (MbCD) and its cholesterol-saturated complex (MbCD/C) changed both the kinetic properties of Kv1.3 and steady-state parameters of activation by modifying lipid–protein interactions. [117]
Zn
Zn+2 ion concentration does not modulate Kv1.3 channel kinetics, although other Kv1 channels are affected. [118]
Leukocyte Kv channel Subunits effect Kv1.3
Leukocytes also express several regulatory subunits which may associate with Kv1.3 complexes to enhance diversity and modulate a wide variety of physiological activities. In fact, Kv1.3 channels are able to assemble with Kvβ subunits to form functional Kv channels. Kvβ subunits alter current amplitude and gating, confer rapid inactivation, and promote Kv surface expression. In addition, heterologous expression of Kv1.3 and Kv1.5 with Kvβ subunits in Xenopus oocytes and mammalian cells, dramatically modifies the rate of inactivation and the K+ current density [1525]
Cluster at C-terminus
A Y479MVIEE484 cluster at the C-terminus of the channel was found responsible for efficient Kv1.3 forward trafficking. This domain is highly conserved in neuronal isoforms of the Kv1 family. The di-acidic (E483/484) motif within the Y479MVIEE484 cluster was the main determinant for Kv1.3 surface expression [1976]
Kv1.3 associates with Kv1.5
Kv1.3 associates with Kv1.5, leading to biophysically and pharmacologically distinct channels. They also generate multiple heterotetramers with differential surface expression according to the subunit composition. FRET analysis and pharmacology confirm the presence of functional hybrid channels. Moreover, FRAP analysis revealed higher mobility for hybrid Kv1.3/Kv1.5 than Kv1.3 homotetramers, suggesting that heteromers target to distinct surface micro domains [1527]
Kv1.3 forms heteromeric channels
Kv1.3 subunit can form heteromeric channels with other Kv1 subfamily subunits. Among the subunits demon- strated to form heteromers with Kv1.3 in vivo are Kv.1.1, Kv1.2, and Kv1.6 [374]
Scorpion toxin ADWX-1 is a pore blocker of Kv1.3 channel without affecting its kinetics
Engineered scorpion toxin ADWX-1 is a potent specific inhibitor of Kv1.3. It has the highest affinity in ~pM to Kv1.3 channels. ADWX-1 blocked the Kv1.3 currents without changing the G-V curve and the time constants of activation and inactivation. This means that the inhibition by ADWX-1 only reduces the number of open channels without affecting the channel kinetics. This allows us to explore the Kv1.3 role in T cell by simply changing the number of Kv1.3 channels in applying with different ADWX-1 concentrations [1532]
Nystatin
The concentrations for nystatin and its structural analog, amphotericin B, required to produce half maximal inhibition (IC50) of the current were estimated to be about 3 and 60 microM, respectively. The effects of nystatin on the amplitude and inactivation of Kv1.3 currents were not voltage-dependent. In inside-out patches, tetraethylammonium (TEA) produced a rapid block of Kv1.3 currents upon the onset of a voltage pulse, while the inhibition by nystatin developed slowly. When co-applied with TEA, nystatin potentiated the extent of the TEA-dependent block, and the kinetic effect of nystatin was slowed by TEA. In summary, nystatin, a compound frequently used in perforated patch recordings to preserve intracellular dialyzable components, specifically inhibited the potassium channel Kv1.3 at concentrations well below those required for perforation [1790]
Sphingomyelinase
The sphingomyelinase enzyme was shown to stimulate native K(v)1.3 channels in nonexcitable human T lymphocytes [1947]
Immunorepressants associated with Kv1.3 modulation
- A mutation of the ShK peptide can enhance its specificity for Kv1.3 and act as selective blocker in the treatment of autoimmune diseases [1948]
- Dihydropyridine Ca(2+) channel blockers, such as nifedipine and benidipine, exert inhibitory effects on thymocyte Kv1.3-channel currents [1951]
- Curcumin was shown to inhibit proinflammatory cytokine secretion of T(EM) cells by blocking of hKv1.3 [1953]
- Clofazimine, Psora-4 and PAP-1 present a therapeutic potential for treatment of leukemia due to their inhibitory effects on Kv1.3 channels [1955]
- Clarithromycin exerts inhibitory effects on thymocyte Kv1.3-channel currents [1957]
- 18β-Glycyrrhetinic acid potently inhibits Kv1.3 channels and T cell activation in Jurkat T cells [1959]
- NMDAR antagonists likely exert their immunosuppressant effect on T cells through the mediation by Kv1.3 and KCa3.1 channels [1961]
- The scorpion toxin analogue HsTX1, a potent and selective Kv1.3 blocker, may be a potential therapeutic for autoimmune diseases [1962]
- Statins modulate thymocytes via the inhibition of Kv1.3-channels [1964]
- PAP-1 inhibition of Kv1.3 was shown to be effective in psoriatic disease in the mouse model [1966]
- Acacetin blocking of the Kv1.3 channel inhibited human T cell activation [1967]
- Xanthohumol and Isoxanthohumol inhibition of Kv1.3 channels was associated with antiproliferative and proapoptotic effect in lymphocytes [1968]
- Cortisone and hydrocortisone are supposed to exert their immunerepressive effects in part by inhibition of Kv1.3 currents via a non-genomic mechanism [1969]
- Diltiazem and verapamil, known as Ca channel blockers, exert inhibitory effects on Kv1.3 in lymphocytes [1972]
- Telmisartan blocked the increased Kv1.3 and KCa3.1 expression in T cells of patients with hypertension [1973]
- Lovastatin exerts immunodulatory properties by blocking of Kv1.3 channels [1975]
EV37 scorpion toxins
Ev37 scorpion toxins elicit cytolytic effects by interaction with Kv1.3 channels. Since they are not effectively blocking Kv1.1 and Kv1.2 channels, Ev37 peptides have been suggested as a new type of selective Kv1.3 channel blockers [1985]
MMP-23 prodomain
The prodomain of the matrix metalloprotease MMP-23 was shown to suppress KV1.3 by intracellular trapping. A proccess not obeserved for KV1.2 channels. The overlap of expression with colorectal cancers indicates a possible involvment of the MMP-23 prodomain and Kv1.3 in this pathology [1986]
Hetlaxin
Hetlaxin, a scorpion toxin from the venom of Heterometrus laoticus, interacts with voltage-gated potassium channel Kv1.3. [1987]
Capsid protein VP1
Parvovirus B19 capsid protein VP1 inhibits Kv1.3 and Kv1.5 channels in host cells. This inhibition was shown to be linked to PLA-dependent formation of lysophosphatidylcholine [1988]
Human β-defensin 2
human β-defensin 2 (hBD2) was reported as a novel modulator of Kv1.3 channels. The hBD2 modulation of Kv1.3 channels involves its interaction with a novel receptor site at the S1-S2 linker. hBD2 positively shifts the conductance-voltage curve of hKv1.3 channels by 10.5 mV and increases its activation time [1989]
BF9 peptide
BF9 is a snake peptide that exhibits a unique bifunctionality of Kv1.3 channel and serine protease inhibiting properties. It is therefore the first reported Kunitz-type snake toxin with protease and potassium channel inhibiting properties [1990]
Loureirin B
Loureirin B a component of the herbal medicine Sanguis draxonis is a blocker of Kv1.3 channels. Its interacting with amino acid residues in the Kv1.3 selectivity filter [1991]
Scorpion toxin Ctri9577
The potent Kv1.3 blocking scorpian toxin Ctri9577, has been revealed as being a novel gating modifier of Kv4.3 channels, as well [1992]
Margatoxin
Margatoxin is a non-selective inhibitor of human Kv1.3 K+ channels that also inhibits Kv1.1 and Kv1.2 channels[1994]
Stichodactyla helianthus toxin
Toxins from Stichodactyla helianthus were described as a potent and selective blockers of the voltage-gated potassium channel Kv1.3. [1995]
Plectasin
The fungal toxin plectasin interacts with the outer pore region of Kv1.3 channels [1996]
Alpha Klotho
The β-glucuronidase enzyme Alpha Klotho was reported to enhance Kv1.3 channel abundance and Kv1.3 currents [1997]
Human α-defensin
Human α-defensins were shown to be novel endogenous inhibitors of Kv1.3 channels [1998]
References
Pereira LE
et al.
Pharmacokinetics, toxicity, and functional studies of the selective Kv1.3 channel blocker 5-(4-phenoxybutoxy)psoralen in rhesus macaques.
Exp. Biol. Med. (Maywood),
2007
Nov
, 232 (1338-54).
Hajdu P
et al.
Cholesterol modifies the gating of Kv1.3 in human T lymphocytes.
Pflugers Arch.,
2003
Mar
, 445 (674-82).
Teisseyre A
et al.
The modulatory effect of zinc ions on voltage-gated potassium currents in cultured rat hippocampal neurons is not related to Kv1.3 channels.
J. Physiol. Pharmacol.,
2007
Dec
, 58 (699-715).
Choi JS
et al.
Mechanism of fluoxetine block of cloned voltage-activated potassium channel Kv1.3.
J. Pharmacol. Exp. Ther.,
1999
Oct
, 291 (1-6).
Feske S
et al.
A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients.
J. Exp. Med.,
2005
Sep
5
, 202 (651-62).
Glutamate levels and activity of the T cell voltage-gated potassium Kv1.3 channel in patients with systemic lupus erythematosus.
Arthritis Rheum., 2008 May , 58 (1445-50).
Douglass J
et al.
Characterization and functional expression of a rat genomic DNA clone encoding a lymphocyte potassium channel.
J. Immunol.,
1990
Jun
15
, 144 (4841-50).
Bielanska J
et al.
Voltage-dependent potassium channels Kv1.3 and Kv1.5 in human fetus.
Cell. Physiol. Biochem.,
2010
, 26 (219-26).
Gazula VR
et al.
Localization of Kv1.3 channels in presynaptic terminals of brainstem auditory neurons.
J. Comp. Neurol.,
2010
Aug
15
, 518 (3205-20).
Bähring R
et al.
Differential modulation of Kv1 channel-mediated currents by co-expression of Kvbeta3 subunit in a mammalian cell-line.
Mol. Membr. Biol.,
2004 Jan-Feb
, 21 (19-25).
Chandy KG
et al.
K+ channels as targets for specific immunomodulation.
Trends Pharmacol. Sci.,
2004
May
, 25 (280-9).
Freedman BD
et al.
Evidence for voltage modulation of IL-2 production in mitogen-stimulated human peripheral blood lymphocytes.
J. Immunol.,
1992
Dec
15
, 149 (3784-94).
Leonard RJ
et al.
Selective blockers of voltage-gated K+ channels depolarize human T lymphocytes: mechanism of the antiproliferative effect of charybdotoxin.
Proc. Natl. Acad. Sci. U.S.A.,
1992
Nov
1
, 89 (10094-8).
Lin CS
et al.
Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation.
J. Exp. Med.,
1993
Mar
1
, 177 (637-45).
Wulff H
et al.
The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS.
J. Clin. Invest.,
2003
Jun
, 111 (1703-13).
Wulff H
et al.
K+ channel expression during B cell differentiation: implications for immunomodulation and autoimmunity.
J. Immunol.,
2004
Jul
15
, 173 (776-86).
Schmitz A
et al.
Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases.
Mol. Pharmacol.,
2005
Nov
, 68 (1254-70).
MacKinnon R
Determination of the subunit stoichiometry of a voltage-activated potassium channel.
Nature,
1991
Mar
21
, 350 (232-5).
Choi KL
et al.
Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels.
Proc. Natl. Acad. Sci. U.S.A.,
1991
Jun
15
, 88 (5092-5).
Zhu J
et al.
N-glycosylation promotes the cell surface expression of Kv1.3 potassium channels.
FEBS J.,
2012
Aug
, 279 (2632-44).
Comes N
et al.
The voltage-dependent K(+) channels Kv1.3 and Kv1.5 in human cancer.
Front Physiol,
2013
, 4 (283).
Koshy S
et al.
Blocking Kv1.3 channels inhibits Th2 lymphocyte function and treats a rat model of asthma.
J. Biol. Chem.,
2014
Mar
18
, ().
Vicente R
et al.
Kv1.5 association modifies Kv1.3 traffic and membrane localization.
J. Biol. Chem.,
2008
Mar
28
, 283 (8756-64).
Toth A
et al.
Functional consequences of Kv1.3 ion channel rearrangement into the immunological synapse.
Immunol. Lett.,
2009
Jun
30
, 125 (15-21).
Wu X
et al.
Effect of the Kv1.3 voltage-gated potassium channel blocker PAP-1 on the initiation and progress of atherosclerosis in a rat model.
Heart Vessels,
2014
Jan
19
, ().
Beeton C
et al.
Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases.
Proc. Natl. Acad. Sci. U.S.A.,
2006
Nov
14
, 103 (17414-9).
Nicolaou SA
et al.
Altered dynamics of Kv1.3 channel compartmentalization in the immunological synapse in systemic lupus erythematosus.
J. Immunol.,
2007
Jul
1
, 179 (346-56).
Hou P
et al.
Physiological role of kv1.3 channel in T lymphocyte cell investigated quantitatively by kinetic modeling.
PLoS ONE,
2014
, 9 (e89975).
Szabò I
et al.
A novel potassium channel in lymphocyte mitochondria.
J. Biol. Chem.,
2005
Apr
1
, 280 (12790-8).
Kremer W
et al.
1.2 Å X-ray structure of the renal potassium channel Kv1.3 T1 domain.
Protein J.,
2013
Oct
, 32 (533-42).
Liu HL
et al.
Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6.
J. Biomol. Struct. Dyn.,
2005
Feb
, 22 (387-98).
Wang J
et al.
Targeting potassium channels Kv1.3 and KC a 3.1: routes to selective immunomodulators in autoimmune disorder treatment?
Pharmacotherapy,
2013
May
, 33 (515-28).
Mullen KM
et al.
Potassium channels Kv1.3 and Kv1.5 are expressed on blood-derived dendritic cells in the central nervous system.
Ann. Neurol.,
2006
Jul
, 60 (118-27).
Hahn SJ
et al.
Inhibition by nystatin of Kv1.3 channels expressed in Chinese hamster ovary cells.
Neuropharmacology,
1996
, 35 (895-901).
Leanza L
et al.
Correlation between potassium channel expression and sensitivity to drug-induced cell death in tumor cell lines.
Curr. Pharm. Des.,
2013
May
16
, ().
Cerni FA
et al.
Electrophysiological characterization of Ts6 and Ts7, K⁺ channel toxins isolated through an improved Tityus serrulatus venom purification procedure.
Toxins (Basel),
2014
Mar
, 6 (892-913).
Tucker K
et al.
Glucose sensitivity of mouse olfactory bulb neurons is conveyed by a voltage-gated potassium channel.
J. Physiol. (Lond.),
2013
May
15
, 591 (2541-61).
Liu J
et al.
HIV-1 Tat protein increases microglial outward K(+) current and resultant neurotoxic activity.
PLoS ONE,
2013
, 8 (e64904).
Duque A
et al.
Expression of Kv1.3 potassium channels regulates density of cortical interneurons.
Dev Neurobiol,
2013
Nov
, 73 (841-55).
Combs DJ
et al.
Tuning voltage-gated channel activity and cellular excitability with a sphingomyelinase.
J. Gen. Physiol.,
2013
Oct
, 142 (367-380).
Rashid MH
et al.
A potent and selective peptide blocker of the Kv1.3 channel: prediction from free-energy simulations and experimental confirmation.
PLoS ONE,
2013
, 8 (e78712).
Rashid MH
et al.
A potent and selective peptide blocker of the Kv1.3 channel: prediction from free-energy simulations and experimental confirmation.
PLoS ONE,
2013
, 8 (e78712).
Toldi G
et al.
The effects of Kv1.3 and IKCa1 potassium channel inhibition on calcium influx of human peripheral T lymphocytes in rheumatoid arthritis.
Immunobiology,
2013
Mar
, 218 (311-6).
Kazama I
et al.
Benidipine persistently inhibits delayed rectifier K(+)-channel currents in murine thymocytes.
Immunopharmacol Immunotoxicol,
2013
Feb
, 35 (28-33).
Yang Y
et al.
Specific Kv1.3 blockade modulates key cholesterol-metabolism-associated molecules in human macrophages exposed to ox-LDL.
J. Lipid Res.,
2013
Jan
, 54 (34-43).
Lian YT
et al.
Curcumin serves as a human kv1.3 blocker to inhibit effector memory T lymphocyte activities.
Phytother Res,
2013
Sep
, 27 (1321-7).
Hu L
et al.
Blockade of Kv1.3 potassium channels inhibits differentiation and granzyme B secretion of human CD8+ T effector memory lymphocytes.
PLoS ONE,
2013
, 8 (e54267).
Leanza L
et al.
Clofazimine, Psora-4 and PAP-1, inhibitors of the potassium channel Kv1.3, as a new and selective therapeutic strategy in chronic lymphocytic leukemia.
Leukemia,
2013
Aug
, 27 (1782-5).
Hautz T
et al.
Targeting the Kv1.3 potassium channel for immunosuppression in vascularized composite allotransplantation - a pilot study.
Transpl. Int.,
2013
May
, 26 (552-61).
Kazama I
et al.
Differential effects of clarithromycin and azithromycin on delayed rectifier K(+)-channel currents in murine thymocytes.
Pharm Biol,
2013
Jun
, 51 (760-5).
Szilágyi O
et al.
The role of PSD-95 in the rearrangement of Kv1.3 channels to the immunological synapse.
Pflugers Arch.,
2013
Sep
, 465 (1341-53).
Fu XX
et al.
18β-Glycyrrhetinic acid potently inhibits Kv1.3 potassium channels and T cell activation in human Jurkat T cells.
J Ethnopharmacol,
2013
Jul
9
, 148 (647-54).
Ueyama A
et al.
Kv1.3 blockers ameliorate allergic contact dermatitis by preferentially suppressing effector memory T cells in a rat model.
Clin. Exp. Dermatol.,
2013
Dec
, 38 (897-903).
Kahlfuß S
et al.
Immunosuppression by NMDA-Receptor Antagonists is Mediated Through Inhibition of Kv1.3 and KCa3.1 Channels in T cells.
Mol. Cell. Biol.,
2013
Dec
16
, ().
Rashid MH
et al.
A potent and Kv1.3-selective analogue of the scorpion toxin HsTX1 as a potential therapeutic for autoimmune diseases.
Sci Rep,
2014
, 4 (4509).
Koch Hansen L
et al.
Expression of T-cell KV1.3 potassium channel correlates with pro-inflammatory cytokines and disease activity in ulcerative colitis.
J Crohns Colitis,
2014
Nov
, 8 (1378-91).
Kazama I
et al.
HMG-CoA reductase inhibitors pravastatin, lovastatin and simvastatin suppress delayed rectifier K(+)-channel currents in murine thymocytes.
Pharmacol Rep,
2014
Aug
, 66 (712-7).
Folyovich A
et al.
Kv1.3 lymphocyte potassium channel inhibition as a potential novel therapeutic target in acute ischemic stroke.
CNS Neurol Disord Drug Targets,
2014
, 13 (801-6).
Kundu-Raychaudhuri S
et al.
Kv1.3 in psoriatic disease: PAP-1, a small molecule inhibitor of Kv1.3 is effective in the SCID mouse psoriasis--xenograft model.
J. Autoimmun.,
2014
Dec
, 55 (63-72).
Zhao N
et al.
Acacetin blocks kv1.3 channels and inhibits human T cell activation.
Cell. Physiol. Biochem.,
2014
, 34 (1359-72).
Gąsiorowska J
et al.
Inhibition of Kv1.3 Channels in Human Jurkat T Cells by Xanthohumol and Isoxanthohumol.
J. Membr. Biol.,
2015
Aug
, 248 (705-11).
Yu J
et al.
Cortisone and hydrocortisone inhibit human Kv1.3 activity in a non-genomic manner.
Naunyn Schmiedebergs Arch. Pharmacol.,
2015
Jun
, 388 (653-61).
Wang LP
et al.
The expression and functional evidence for voltage-dependent potassium channel Kv1.3 in lymphocytes during aging in spontaneously hypertensive rats.
Int J Clin Exp Med,
2015
, 8 (2506-15).
Zhu H
et al.
Kv1.3 channel blockade enhances the phagocytic function of RAW264.7 macrophages.
Sci China Life Sci,
2015
Sep
, 58 (867-75).
Baba A
et al.
Suppressive effects of diltiazem and verapamil on delayed rectifier K(+)-channel currents in murine thymocytes.
Pharmacol Rep,
2015
Oct
, 67 (959-64).
Zhang Q
et al.
Potassium channel changes of peripheral blood T-lymphocytes from Kazakh hypertensive patients in Northwest China and the inhibition effect towards potassium channels by telmisartan.
Kardiol Pol,
2015
Oct
27
, ().
Zhang S
et al.
Blockage of KCa3.1 and Kv1.3 channels of the B lymphocyte decreases the inflammatory monocyte chemotaxis.
Int. Immunopharmacol.,
2016
Jan
18
, 31 (266-271).
Zhao N
et al.
Lovastatin blocks Kv1.3 channel in human T cells: a new mechanism to explain its immunomodulatory properties.
Sci Rep,
2015
, 5 (17381).
Martínez-Mármol R
et al.
A non-canonical di-acidic signal at the C-terminus of Kv1.3 determines anterograde trafficking and surface expression.
J. Cell. Sci.,
2013
Dec
15
, 126 (5681-91).
Delaney E
et al.
Determinants of pore folding in potassium channel biogenesis.
Proc. Natl. Acad. Sci. U.S.A.,
2014
Mar
25
, 111 (4620-5).
Zhu J
et al.
The Kv1.3 potassium channel is localized to the cis-Golgi and Kv1.6 is localized to the endoplasmic reticulum in rat astrocytes.
FEBS J.,
2014
Aug
, 281 (3433-45).
Hajdu P
et al.
The C-terminus SH3-binding domain of Kv1.3 is required for the actin-mediated immobilization of the channel via cortactin.
Mol. Biol. Cell,
2015
May
1
, 26 (1640-51).
Jang SH
et al.
Nuclear Localization and Functional Characteristics of Voltage-gated Potassium Channel, Kv1.3.
J. Biol. Chem.,
2015
Mar
31
, ().
Spear JM
et al.
Kv1.3 contains an alternative C-terminal ER exit motif and is recruited into COPII vesicles by Sec24a.
BMC Biochem.,
2015
, 16 (16).
Martínez-Mármol R
et al.
Unconventional EGF-induced ERK1/2-mediated Kv1.3 endocytosis.
Cell. Mol. Life Sci.,
2015
Nov
5
, ().
Jiménez-Pérez L
et al.
Molecular Determinants of Kv1.3 Potassium Channels-Induced Proliferation.
J. Biol. Chem.,
2015
Dec
10
, ().
Kudryashova KS
et al.
Fluorescent system based on bacterial expression of hybrid KcsA channels designed for Kv1.3 ligand screening and study.
Anal Bioanal Chem,
2013
Mar
, 405 (2379-89).
Feng J
et al.
Expression and characterization of a novel scorpine-like peptide Ev37, from the scorpion Euscorpiops validus.
Protein Expr. Purif.,
2013
Mar
, 88 (127-33).
Nguyen HM
et al.
Intracellular trafficking of the KV1.3 potassium channel is regulated by the prodomain of a matrix metalloprotease.
J. Biol. Chem.,
2013
Mar
1
, 288 (6451-64).
Anh HN
et al.
Hetlaxin, a new toxin from the Heterometrus laoticus scorpion venom, interacts with voltage-gated potassium channel Kv1.3.
Dokl. Biochem. Biophys.,
2013 Mar-Apr
, 449 (109-11).
Ahmed M
et al.
Down-regulation of K⁺ channels by human parvovirus B19 capsid protein VP1.
Biochem. Biophys. Res. Commun.,
2014
Aug
8
, 450 (1396-401).
Feng J
et al.
Kv Channel S1-S2 Linker Working as a Binding Site of Human β-Defensin 2 for Channel Activation Modulation.
J. Biol. Chem.,
2015
Jun
19
, 290 (15487-95).
Yang W
et al.
BF9, the first functionally characterized snake toxin peptide with Kunitz-type protease and potassium channel inhibiting properties.
J. Biochem. Mol. Toxicol.,
2014
Feb
, 28 (76-83).
Yin S
et al.
Loureirin B, an essential component of Sanguis Draxonis, inhibits Kv1.3 channel and suppresses cytokine release from Jurkat T cells.
Cell Biosci,
2014
, 4 (78).
Xie C
et al.
Kv1.3 potassium channel-blocking toxin Ctri9577, novel gating modifier of Kv4.3 potassium channel from the scorpion toxin family.
Biochem. Biophys. Res. Commun.,
2014
Feb
14
, 444 (406-10).
Sabogal-Arango A
et al.
Computational Insights of the Interaction among Sea Anemones Neurotoxins and Kv1.3 Channel.
Bioinform Biol Insights,
2014
, 8 (73-81).
Bartok A
et al.
Margatoxin is a non-selective inhibitor of human Kv1.3 K(+) channels.
Toxicon,
2014
Sep
, 87 (6-16).
Chang SC
et al.
N-Terminally extended analogues of the K⁺ channel toxin from Stichodactyla helianthus as potent and selective blockers of the voltage-gated potassium channel Kv1.3.
FEBS J.,
2015
Jun
, 282 (2247-59).
Xiang F
et al.
Plectasin, first animal toxin-like fungal defensin blocking potassium channels through recognizing channel pore region.
Toxins (Basel),
2015
Jan
, 7 (34-42).
Almilaji A
et al.
Regulation of the voltage gated K channel Kv1.3 by recombinant human klotho protein.
Kidney Blood Press. Res.,
2014
, 39 (609-22).
Xie Z
et al.
Human α-defensins are immune-related Kv1.3 channel inhibitors: new support for their roles in adaptive immunity.
FASEB J.,
2015
Oct
, 29 (4324-33).
Somodi S
et al.
Analysis of the K+ current in human CD4+ T lymphocytes in hypercholesterolemic state.
Cell. Immunol.,
2013
Jan
, 281 (20-6).
Kan XH
et al.
Kv1.3 potassium channel mediates macrophage migration in atherosclerosis by regulating ERK activity.
Arch. Biochem. Biophys.,
2016
Feb
1
, 591 (150-6).
Ladjel-Mendil A
et al.
Neuropathophysiological effect and immuno-inflammatory response induced by kaliotoxin of androctonus scorpion venom.
Neuroimmunomodulation,
2013
, 20 (99-106).
Peng Y
et al.
Blockade of Kv1.3 channels ameliorates radiation-induced brain injury.
Neuro-oncology,
2014
Apr
, 16 (528-39).
Wang J
et al.
Effect of methamphetamine on the microglial damage: role of potassium channel Kv1.3.
PLoS ONE,
2014
, 9 (e88642).
Wu J
et al.
Voltage-gated potassium channel Kv1.3 is highly expressed in human osteosarcoma and promotes osteosarcoma growth.
Int J Mol Sci,
2013
, 14 (19245-56).
Cidad P
et al.
K+ channels expression in hypertension after arterial injury, and effect of selective Kv1.3 blockade with PAP-1 on intimal hyperplasia formation.
Cardiovasc Drugs Ther,
2014
Dec
, 28 (501-11).
Zhao J
et al.
Gene identification of potential malignant parathyroid tumors phenotype in Chinese population.
Endocr. J.,
2014
, 61 (597-605).
Cidad P
et al.
Kv1.3 channels modulate human vascular smooth muscle cells proliferation independently of mTOR signaling pathway.
Pflugers Arch.,
2015
Aug
, 467 (1711-22).
Johnson MC
et al.
Odor enrichment sculpts the abundance of olfactory bulb mitral cells.
Neurosci. Lett.,
2013
Apr
29
, 541 (173-8).
Dufour MA
et al.
Somatodendritic ion channel expression in substantia nigra pars compacta dopaminergic neurons across postnatal development.
J. Neurosci. Res.,
2014
Aug
, 92 (981-99).
Zhou YY
et al.
Psora-4, a Kv1.3 Blocker, Enhances Differentiation and Maturation in Neural Progenitor Cells.
CNS Neurosci Ther,
2015
Jul
, 21 (558-67).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/3/ , accessed on 2026 Feb 23