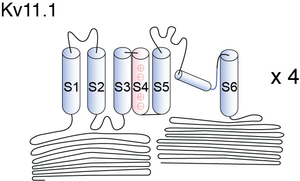
Kv11.1
Description: potassium voltage-gated channel, subfamily H (eag-related), member 2 Gene: Kcnh2 Alias: Kv11.1, ERG1, HERG, LQT2, SQT1, HERG1, KCNH2
Kv11.1, encoded by the gene KCNH2, and also known as ERG1; HERG; LQT2; SQT1; HERG1, is a voltage-activated potassium channel belonging to the eag family. Kv11.1 is responsible for the rapid delayed rectifier current, IKr, in the heart that is essential for repolarization of the cardiac action potential and normal cardiac electrical activity and rhythm. Mutations in this gene can cause long QT syndrome type 2 (LQT2). NCBI
Experimental data
Rat Kv11.1 gene in CHO host cells datasheet |
||
|
Click for details 
15 °Cshow 94 cells |
Click for details 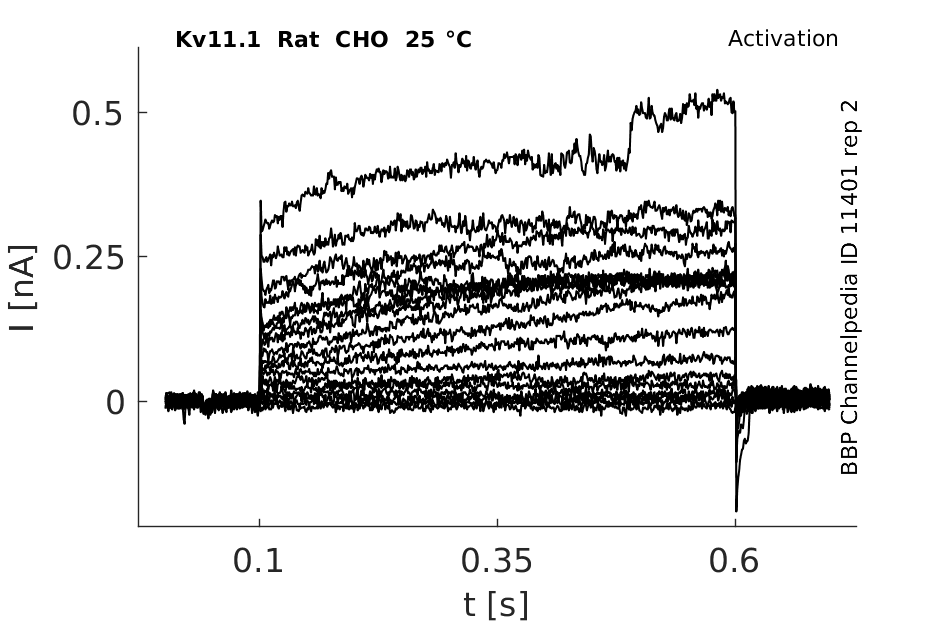
25 °Cshow 137 cells |
Click for details 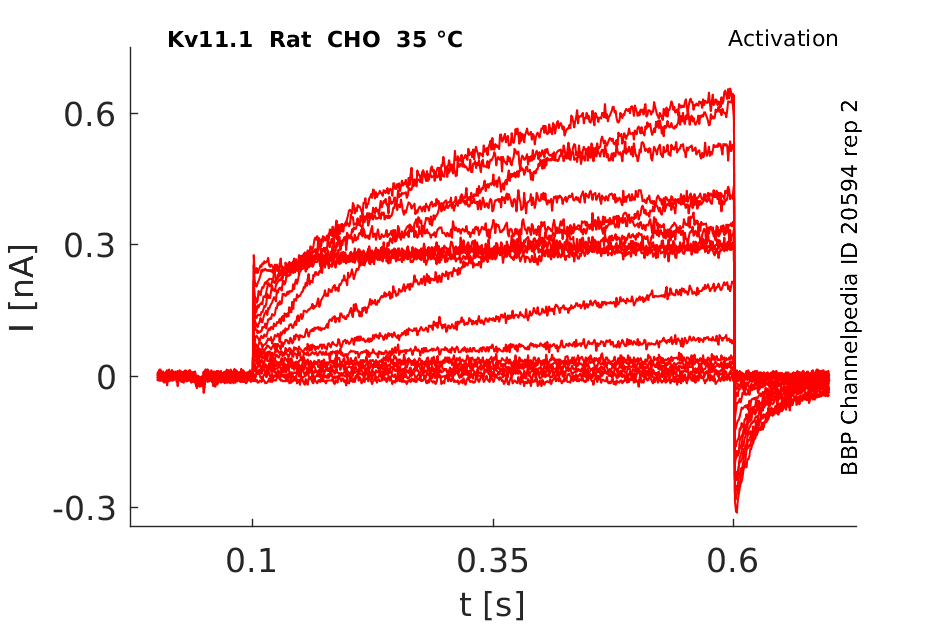
35 °Cshow 151 cells |
Rat Kv11.1 gene in HEK host cells |
||
|
Click for details 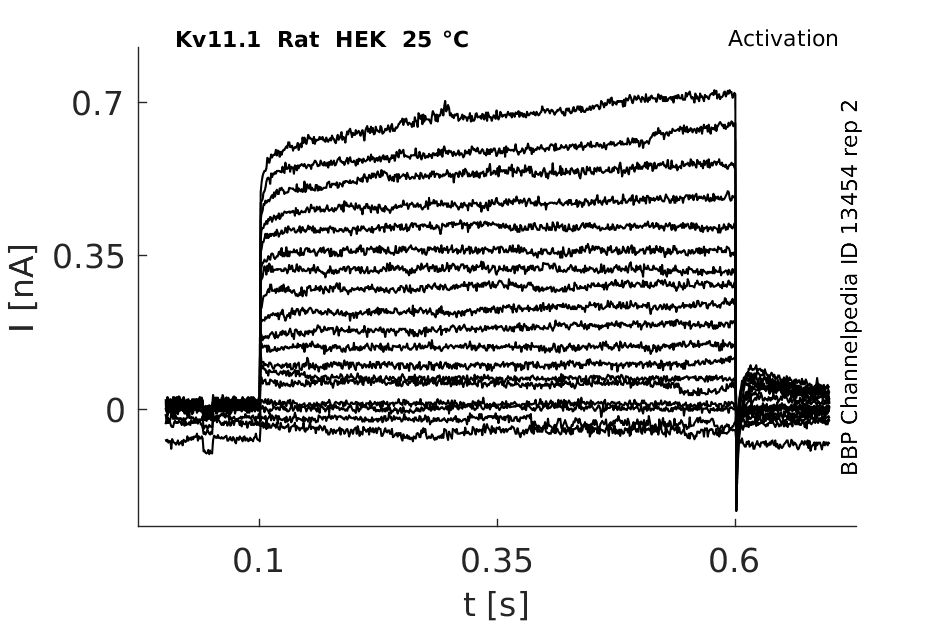
25 °Cshow 44 cells |
||
Rat Kv11.1 gene in CV1 host cells |
||
|
Click for details 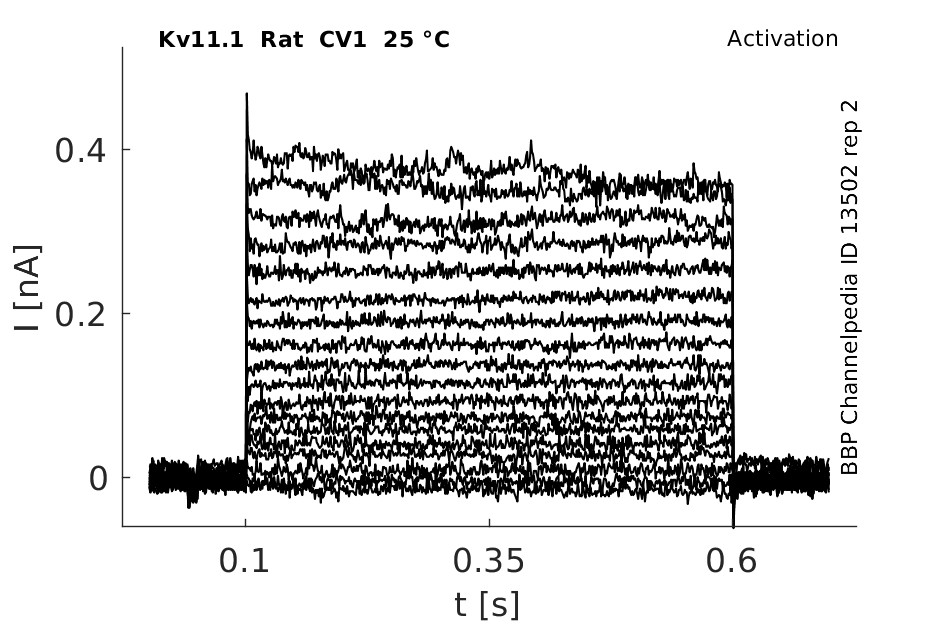
25 °Cshow 64 cells |
||
The human KCNH2 (hERG1) gene is located on the long (q) arm of chromosome 7 at position 36.1 (between base pairs 150,642,043 to 150,675,401) and consists of 15 exons ( Fig. 1A). [1518]
Transcript variants encoding distinct isoforms of Kv11.1 have been identified.
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_000238.4 | 4292 | |
| Mouse | NM_013569.2 | 4221 | |
| Rat | NM_053949.1 | 3889 |
Protein Synthesis
The Kv11.1 protein is initially synthesized in the endoplasmatic reticulum (ER) as the core-glycosylated precursor form and becomes fully glycosylated in the Golgi apparatus from where the mature form is translocated to the plasma membrane [1518]
Isoforms
Post-Translational Modifications
Visual Representation of Kv11.1 Structure
Methodology for visual representation of structure available here
Cartoon and Crystal Structure

Kv11.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
In contrast to other Kv channels, hERG channels display unusual gating characteristics, which include slow activation and rapid voltage-dependent inactivation [1515]. With inactivation time constants (in the order of ms) some 1–2 orders of magnitude smaller than the activation time constants (in a range up to hundreds of ms) at the same potential [1508]
Biophysical Properties of HERG in CHO-K1
Whole-cell voltage clamp recordings of HERG-transfected cells revealed currents (‘HERG currents') with biophysical properties similar to those reported by previous investigators. Current-voltage relations and activation kinetics were determined using step depolarizations to potentials between −35 and +25 mV from a holding potential of −55 mV (Figure 1b). Activating current amplitude increased with voltage to a maximum at −5 mV (Figure 1c), then declined at more positive potentials due to strong inward rectification.
Effects of Temperature on hERG channel in CHO cells

Inward Rectification
The different physiological roles of erg channels are enabled by their peculiar gating [778]. Although they are voltage-gated K+ channels constructed of subunits with six transmembrane domains, functionally, erg channels are inward rectifiers. This inward rectification is due to fast inactivation kinetics combined with slow activation as well as fast recovery from inactivation combined with slow deactivation [784], [785], [786], [787], [788].
OAG
Stimulation of PKC with 1-oleoyl 2-acetylglycerol (OAG), decreased current amplitudes in a concentration dependent manner (pIC50 = 5.9 ± 0.1, n ≥ 4) [1520]
β9-strand Replacement
Replacement of the predicted β9-strand in Kv11.1 cNBH domain (860-FNL-862) with alanine residues not only destabilizes the open state relative to the closed state (fig2), it also destabilizes the inactivated state relative to the open state [1522]
RPR260243 has been designated as a type 1 hERG channel activator (Perry et al. 2009). This small molecule enhances current by attenuating inactivation and severely slowing the rate of channel closure. By contrast, PD118057 binds closer to the selectivity filter, forming intersubunit interactions between the pore helix and S6 that shifts the voltage dependence of inactivation to more depolarized potentials [1523]
Temperature effect on mutated hERG expression
Impact of temperature and voltage protocols in HERG channels
The impression from previous studies using oocytes and mammalian cell lines was that the changes in the gating behaviors and current density of HERG channel at higher temperature poise channels more sensitive to HERG blockers such that lower IC50 values were more likely to be obtained at physiological temperatures. by changing temperature from 22 °C to 35 °C, the alteration in potency of the compounds tested was diversified: for E-4031 the potency was unchanged, for ketoconazole it was slightly increased, and for astemizole it was decreased. The reason for the lower apparent potency of astemizole at near-physiological temperature is not clear. It is possible that: (1) astemizole blocks the opened channels more powerfully than it blocks the inactivated channels, and at the high temperature, acceleration of transition from the open state to inactivated state leads to a shortening of period that the drug interacts with the opened channels; and (2) astemizole has less time to interacts with channels at the open/inactivated states at 35 °C since the duration of Vt in high-temperature protocols (2 s) is much shorter than that in the standard protocol (5 s). These results suggest that the mechanisms underlying the impact of temperature may vary greatly, depending on the alterations in drug–channel interactions at different temperatures [1739]
Single Channel of Herg in CHO cells

MARKOV MODEL OF Kv11.1
The continuous-time Markov state model was phrased as a system of non-autonomous ordinary differential equations along with an algebraic equation representing the conservation of states property of Markov chains. Each state transition is described by a forward rate: α =α0 exp[zαVm/(RT/F)] and a backward rate: β =β0exp[-zβVm/(RT/F)], where R is the universal gas constant, T is the absolute temperature and F is Faraday's constant [1701]
MARKOV MODEL OF DRUG-BINDING TO Kv11.1
HODGKIN AND HUXLEY MODEL and MARKOV MODEL FOR HERG
Models of hERG gating. A: Markov state descriptions of hERG kinetics: (i) linear scheme, (ii) branched scheme, (iii) subunit scheme, and (iv) relaxed-activated state scheme. B: action potentials (top panel) and Kv11.1 currents (bottom panel) simulated using different hERG gating models. In each case, the Hodgkin-Huxley formulation of the IKr component in the ten Tusscher description of the ventricular action potential was replaced with the model shown. For comparison, the maximum IKr conductance was equivalent in each case. Simulations were carried out at 37°C. Where models were derived from data at different temperatures, rate constants were corrected using a Q10 of 3.3 [1733]
Expression of hERG comparison to other cardiac channel
Compared to Kir2.1 and hEAG, hERG is twice and four times, respectively, more broadly expressed across tissues, tumors, and developmental stages. Importantly, KCNQ1 also exhibits similar levels of expression to hERG in these three EST profile sets. We also caution that these data may represent a conservative estimate, as some examples of negative expression in the hERG EST profile, such as breast tumors, contradict existing functional evidence in these cells
Expression in Rat Brain
All three transcripts are expressed throughout the rat brain: in the olfactory bulb, and erg1 and erg3 are co-expressed in the reticular thalamic nucleus, cerebral cortex, cerebellum and hippocampus [327].
erg subunits can be expressed in different combinations in individual rat lactotroph cells [781].
Transcript location
Transcripts for more than one erg subunit have been detected in various cell lines: NG108-15 (neuroblastoma, erg1–3, [793]), PC12 (sympa- thetic ganglia neuron, erg1 and erg2, [327]), MMQ (lactotroph, erg1–3, [794]) and GH3/B6 (somatomam- motroph, erg1 and erg2, [795]).
Tissue distribution of herg1
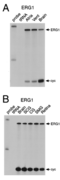
Kv11.1 distribution in Retina
The only study on the localization of Kv11 channel proteins in the retina so far reported an expression of Kv11.1 subunits in somata and primary dendrites of horizontal cells [1734]
Cardiac Repolarization
Human ether-a-go-go-related gene (hERG) potassium channels conduct the rapid component of the delayed rectifier potassium current, IKr, which is crucial for repolarization of cardiac action potentials. Moderate hERG blockade may produce a beneficial class III antiarrhythmic effect. In contrast, a reduction in hERG currents due to either genetic defects or adverse drug effects can lead to hereditary or acquired long QT syndromes characterized by action potential prolongation, lengthening of the QT interval on the surface ECG, and an increased risk for "torsade de pointes" arrhythmias and sudden death [1842]
Volume and Na2+ Regulation
Rat ERG channels have also been identified in the kidney, where they display heterogeneous subcellular localization according to nephron segment66. Here, the channel function may be related to volume regulation and osmotic balance during sodium transport [1730]
Fetal development
In addition to regulating LQTS in adults, hERG, like other potassium channels83, appears to have an important role in development. Data derived from mutational analyses of an Arabian family with frequent miscarriages suggests that homozygous nonsense mutations in the channel may be associated with embryonic lethality15. Functional experiments based on this genetic analysis highlight the nonsense-mediated decay of the hERG transcript and subsequent neonatal arrhythmias as a potential mechanism for this recurrent fetal loss [1731]
Erg (eag-related) channels play critical roles in regulating the resting membrane potential [780],[781], action potential duration [782], spike frequency adaptation and hormone secretion [781]. Due to a Per-Arnt-Sim domain in the N-terminus of erg channel subunits, even a role in O2-sensing has been discussed [783].
Long and Short QT Syndrome and other Diseases
Herg channel is associated with numerous diseases including tumours, Epilepsy, Cardiovascular disease, Schizophrenia, QT syndrome and Muscular dystrophy [1732]
Labour Contractions
Diminished hERG K(+) channel activity facilitates strong human labour contractions but is dysregulated in obese women [1754]
Escitalopram/Citalopram
Several pharmaceutical drugs target the HERG channel current. Two widely used anti-depressants including escitalopram and citalopram blocked HERG currents in a concentration-dependent manner with an IC50 value of 2.6 μM for escitalopram and an IC50 value of 3.2 μM for citalopram [1515]
Lamotrigine and topiramate
Tail currents, which are purely related to hERG currents, were blocked with IC50 and IC20 (the concentrations when 50% and 20% inhibition was obtained compared to control values) of 229 and 21 microM, respectively, for Lamotrigine. A 35% inhibition of tail currents was obtained at Topiramate concentrations of 1000 microM and a 20% inhibition at 87 microM, respectively [1517]
Ranolazine

Mutaion removes hERG block
An S4-S5 linker mutation that allows reactivation of current at hyperpolarized voltages alleviates hERG block, indicating that drugs are trapped in the vestibule by a gate that regulates the permeant path [1519]
Cisapride

NS164
The key residues that seem to interact with NS1643 (1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea) are located on the S5 and S6 segments of adjacent subunits and are situated near the pore helix. In all probability, drug bound to this site interferes with the subtle rearrangement of the pore helix/selectivity filter that is believed to underlie P-type inactivation [1521]
RPR260243
RPR260243 has been designated as a type 1 hERG channel activator (Perry et al. 2009). This small molecule enhances current by attenuating inactivation and severely slowing the rate of channel closure (deactivation).
RPR regulates hERG1 and rERG2 differentially
RPR260243 (RPR) induces voltage-dependent slowing of hERG1 deactivation. A study using site-directed mutagenesis proposed the C-linker domain as key component of slow deactivation in ERG channels and found that residues in the C-linker and the adjacent cyclic nucleotide-binding homology domains are sufficient to explain the different sensitivities of hERG1 and rERG2 to RPR.[2091]
Type 2 Activators
hERG channel activators, such as PD118057, its analogue PD307243, NS1643, A935142 and ICA-105574, act primarily to attenuate inactivation and are designated as type 2 activators. Impaired inactivation occurs through a dual mechanism involving both a shift in the voltage dependence of inactivation to more depolarized membrane potentials and a slowing of the onset rate [1523]
α1A and β adrenoceptor (AR)
IKr and hERG current modulation by α1A and β adrenoceptor (AR) stimulation is blocked by inhibitors of protein kinases. Elevating cAMP to directly activate protein kinase A (PKA) causes a positive shift of activation that is removed when four consensus PKA phosphorylation sites on hERG are mutated. Thus, PKA stimulation alters channel function by a mechanism that requires direct phosphorylation of hERG subunits [1520]
KCNE1 and KCNE2
KCNE1 and KCNE2 are single transmembrane domain proteins that interact with the pore-forming subunits of KCNQ1 and hERG proteins. Whereas KCNE1 subunits are essential components of the IKs channel complex, the role of KCNEs in regulating Kv11.1 function is still a topic of debate. Both KCNE1 and KCNE2 have been shown to associate with hERG and alter gating kinetics of Kv11.1 both in oocytes and mammalian cell lines. KCNE1 antisense oligos also reduce IKr density in the atrial tumor cell line (AT1 cell). A study in horse heart has provided additional evidence that KCNE1 can coimmunoprecipitate with hERG in native tissue [1733]
BmKKx2
hERG potassium channel blockage by scorpion toxin BmKKx2 enhances erythroid differentiation of human leukemia cells K562 [1740]
Chloroquine
Chloroquine also slowed the apparent rate of HERG deactivation, reflecting the inability of drug-bound channels to close [1742]
V625A, Y652A and F656A
These mutations decrease the potency of channel block by MK-499 [1742]
Putative hERG-interacting proteins revealed by yeast two-hybrid technique
The yeast two-hybrid technique was used to reveal interacting proteins for the human ERG protein (Kv11.1). Caveolin-1, FHL2 (zinc finger protein) and PTPN12 (a non-receptor tyrosine phosphatase), as well as eight hERG carboxylic terminal-interacting proteins were were identified.[2092]
References
Wimmers S
et al.
Biophysical properties of heteromultimeric erg K+ channels.
Pflugers Arch.,
2002
Dec
, 445 (423-30).
Papa M
et al.
Expression pattern of the ether-a-gogo-related (ERG) K+ channel-encoding genes ERG1, ERG2, and ERG3 in the adult rat central nervous system.
J. Comp. Neurol.,
2003
Nov
3
, 466 (119-35).
Saganich MJ
et al.
Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain.
J. Neurosci.,
2001
Jul
1
, 21 (4609-24).
Akbarali HI
et al.
Role of HERG-like K(+) currents in opossum esophageal circular smooth muscle.
Am. J. Physiol.,
1999
Dec
, 277 (C1284-90).
Bauer CK
et al.
A functional role of the erg-like inward-rectifying K+ current in prolactin secretion from rat lactotrophs.
Mol. Cell. Endocrinol.,
1999
Feb
25
, 148 (37-45).
Sanguinetti MC
et al.
A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel.
Cell,
1995
Apr
21
, 81 (299-307).
Overholt JL
et al.
HERG-Like potassium current regulates the resting membrane potential in glomus cells of the rabbit carotid body.
J. Neurophysiol.,
2000
Mar
, 83 (1150-7).
Shibasaki T
Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart.
J. Physiol. (Lond.),
1987
Jun
, 387 (227-50).
Smith PL
et al.
The inward rectification mechanism of the HERG cardiac potassium channel.
Nature,
1996
Feb
29
, 379 (833-6).
Spector PS
et al.
Fast inactivation causes rectification of the IKr channel.
J. Gen. Physiol.,
1996
May
, 107 (611-9).
Trudeau MC
et al.
HERG, a human inward rectifier in the voltage-gated potassium channel family.
Science,
1995
Jul
7
, 269 (92-5).
Wang S
et al.
A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes.
J. Physiol. (Lond.),
1997
Jul
1
, 502 ( Pt 1) (45-60).
Bauer CK
et al.
RERG is a molecular correlate of the inward-rectifying K current in clonal rat pituitary cells.
Recept. Channels,
1998
, 6 (19-29).
Shi W
et al.
Identification of two nervous system-specific members of the erg potassium channel gene family.
J. Neurosci.,
1997
Dec
15
, 17 (9423-32).
Warmke JW
et al.
A family of potassium channel genes related to eag in Drosophila and mammals.
Proc. Natl. Acad. Sci. U.S.A.,
1994
Apr
12
, 91 (3438-42).
Wimmers S
et al.
Erg1, erg2 and erg3 K channel subunits are able to form heteromultimers.
Pflugers Arch.,
2001
Jan
, 441 (450-5).
Meves H
et al.
Separation of M-like current and ERG current in NG108-15 cells.
Br. J. Pharmacol.,
1999
Jul
, 127 (1213-23).
Lecchi M
et al.
Isolation of a long-lasting eag-related gene-type K+ current in MMQ lactotrophs and its accommodating role during slow firing and prolactin release.
J. Neurosci.,
2002
May
1
, 22 (3414-25).
Wulfsen I
et al.
Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary.
J. Neuroendocrinol.,
2000
Mar
, 12 (263-72).
Zhang P
et al.
New Aspects of HERG K(+) Channel Function Depending upon Cardiac Spatial Heterogeneity.
PLoS ONE,
2014
, 9 (e72181).
Thouta S
et al.
Proline scan of the HERG channel s6 helix reveals the location of the intracellular pore gate.
Biophys. J.,
2014
Mar
4
, 106 (1057-69).
Chae YJ
et al.
Escitalopram block of hERG potassium channels.
Naunyn Schmiedebergs Arch. Pharmacol.,
2014
Jan
, 387 (23-32).
Danielsson BR
et al.
Effects of the antiepileptic drugs lamotrigine, topiramate and gabapentin on hERG potassium currents.
Epilepsy Res.,
2005
Jan
, 63 (17-25).
Jonsson MK
et al.
Deciphering hERG channels: molecular basis of the rapid component of the delayed rectifier potassium current.
J. Mol. Cell. Cardiol.,
2012
Sep
, 53 (369-74).
Mitcheson JS
et al.
Trapping of a methanesulfonanilide by closure of the HERG potassium channel activation gate.
J. Gen. Physiol.,
2000
Mar
, 115 (229-40).
Cockerill SL
et al.
Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits.
J. Physiol. (Lond.),
2007
Jun
1
, 581 (479-93).
Grunnet M
et al.
Molecular determinants of human ether-à-go-go-related gene 1 (hERG1) K+ channel activation by NS1643.
Mol. Pharmacol.,
2011
Jan
, 79 (1-9).
Ng CA
et al.
C-Terminal β9-Strand of the Cyclic Nucleotide-Binding Homology Domain Stabilizes Activated States of Kv11.1 Channels.
PLoS ONE,
2013
, 8 (e77032).
Perry M
et al.
Revealing the structural basis of action of hERG potassium channel activators and blockers.
J. Physiol. (Lond.),
2010
Sep
1
, 588 (3157-67).
Lu Y
et al.
Effects of premature stimulation on HERG K(+) channels.
J. Physiol. (Lond.),
2001
Dec
15
, 537 (843-51).
Du C
et al.
Ranolazine inhibition of hERG potassium channels: Drug-pore interactions and reduced potency against inactivation mutants.
J. Mol. Cell. Cardiol.,
2014
May
27
, ().
Kang J
et al.
Discovery of a small molecule activator of the human ether-a-go-go-related gene (HERG) cardiac K+ channel.
Mol. Pharmacol.,
2005
Mar
, 67 (827-36).
Zhou Z
et al.
Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects.
J. Biol. Chem.,
1999
Oct
29
, 274 (31123-6).
Mohammad S
et al.
Blockage of the HERG human cardiac K+ channel by the gastrointestinal prokinetic agent cisapride.
Am. J. Physiol.,
1997
Nov
, 273 (H2534-8).
Wang S
et al.
Recent developments in computational prediction of HERG blockage.
Curr Top Med Chem,
2013
Jun
1
, 13 (1317-26).
Babcock JJ
et al.
hERG channel function: beyond long QT.
Acta Pharmacol. Sin.,
2013
Mar
, 34 (329-35).
Bhuiyan ZA
et al.
Recurrent intrauterine fetal loss due to near absence of HERG: clinical and functional characterization of a homozygous nonsense HERG Q1070X mutation.
Heart Rhythm,
2008
Apr
, 5 (553-61).
He FZ
et al.
Current pharmacogenomic studies on hERG potassium channels.
Trends Mol Med,
2013
Apr
, 19 (227-38).
Vandenberg JI
et al.
hERG K(+) channels: structure, function, and clinical significance.
Physiol. Rev.,
2012
Jul
, 92 (1393-478).
Cordeiro S
et al.
Expression pattern of Kv11 (Ether à-go-go-related gene; erg) K+ channels in the mouse retina.
PLoS ONE,
2011
, 6 (e29490).
Yao JA
et al.
Estimation of potency of HERG channel blockers: impact of voltage protocol and temperature.
,
2005 Jul-Aug
, 52 (146-53).
Ma J
et al.
hERG potassium channel blockage by scorpion toxin BmKKx2 enhances erythroid differentiation of human leukemia cells K562.
PLoS ONE,
2013
, 8 (e84903).
Narayana Moorthy NS
et al.
Human ether-a-go-go-related gene channel blockers and its structural analysis for drug design.
Curr Drug Targets,
2013
Jan
1
, 14 (102-13).
Sánchez-Chapula JA
et al.
Molecular determinants of voltage-dependent human ether-a-go-go related gene (HERG) K+ channel block.
J. Biol. Chem.,
2002
Jun
28
, 277 (23587-95).
Parkington HC
et al.
Diminished hERG K(+) channel activity facilitates strong human labour contractions but is dysregulated in obese women.
Nat Commun,
2014
, 5 (4108).
Thomas D
et al.
The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications.
Curr. Pharm. Des.,
2006
, 12 (2271-83).
Chen MX
et al.
Improved functional expression of recombinant human ether-a-go-go (hERG) K+ channels by cultivation at reduced temperature.
BMC Biotechnol.,
2007
, 7 (93).
Gardner A
et al.
C-Linker Accounts for Differential Sensitivity of ERG1 and ERG2 K+ Channels to RPR260243-Induced Slow Deactivation.
Mol. Pharmacol.,
2015
Jul
, 88 (19-28).
Ma Q
et al.
Screening for cardiac HERG potassium channel interacting proteins using the yeast two-hybrid technique.
Cell Biol. Int.,
2014
Feb
, 38 (239-45).
Beattie KA
et al.
Sinusoidal voltage protocols for rapid characterisation of ion channel kinetics.
J. Physiol. (Lond.),
2018
05
15
, 596 (1813-1828).
Lei CL
et al.
Rapid Characterization of hERG Channel Kinetics I: Using an Automated High-Throughput System.
Biophys. J.,
2019
Jul
25
, ().
Lei CL
et al.
Rapid Characterization of hERG Channel Kinetics II: Temperature Dependence.
Biophys. J.,
2019
Jul
25
, ().
Contributors: Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/35/ , accessed on 2026 Mar 01