Kv11.2
Description: potassium voltage-gated channel, subfamily H (eag-related), member 6 Gene: Kcnh6 Alias: Kv11.2, ERG2, HERG2, KCNH6
Kv11.2, encoded by the gene KCNH6 , is a voltage-activated potassium channel belonging to the eag family.
Experimental data
Rat Kv11.2 gene in CHO host cells datasheet |
||
|
Click for details 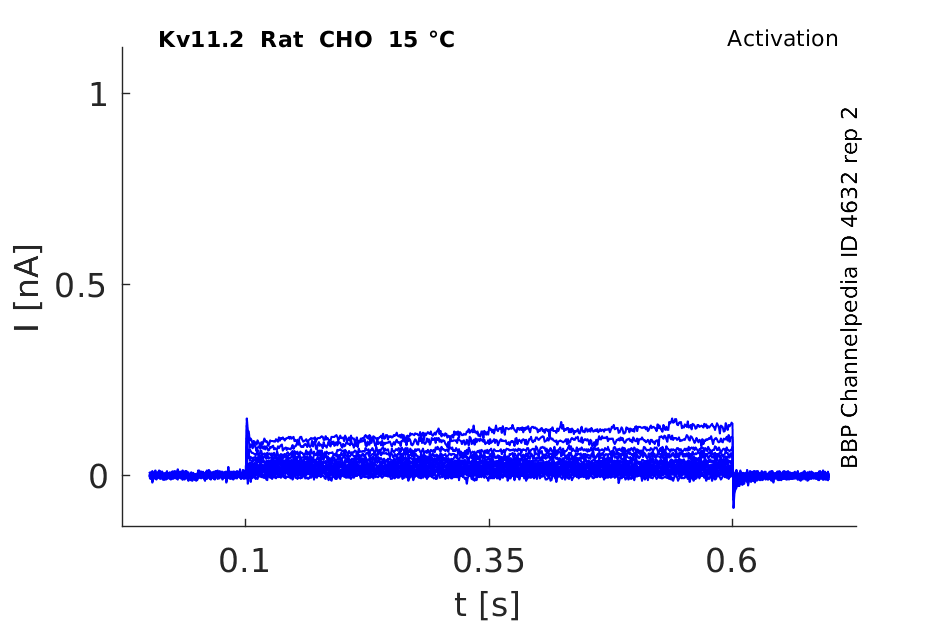
15 °Cshow 106 cells |
Click for details 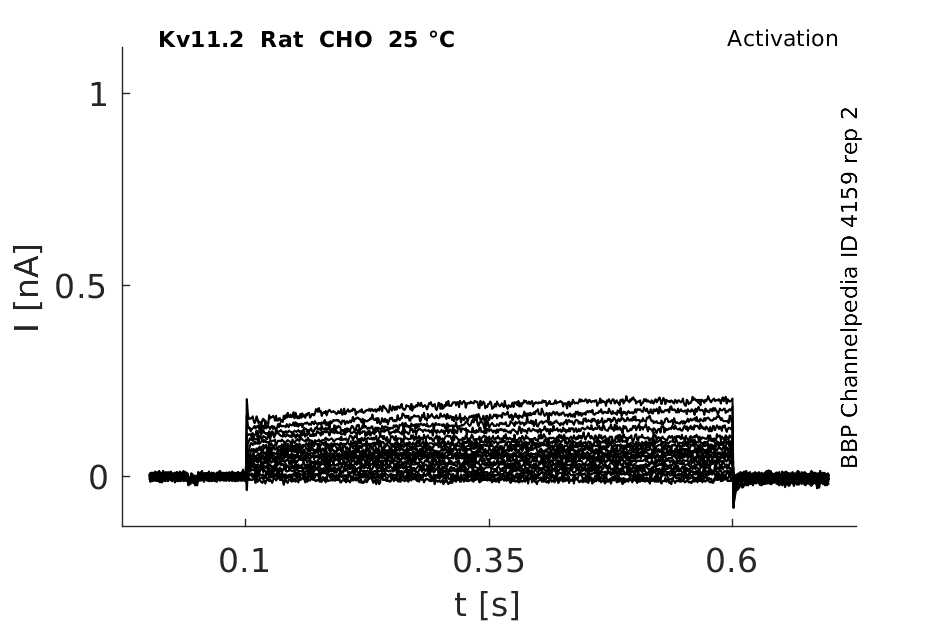
25 °Cshow 70 cells |
Click for details 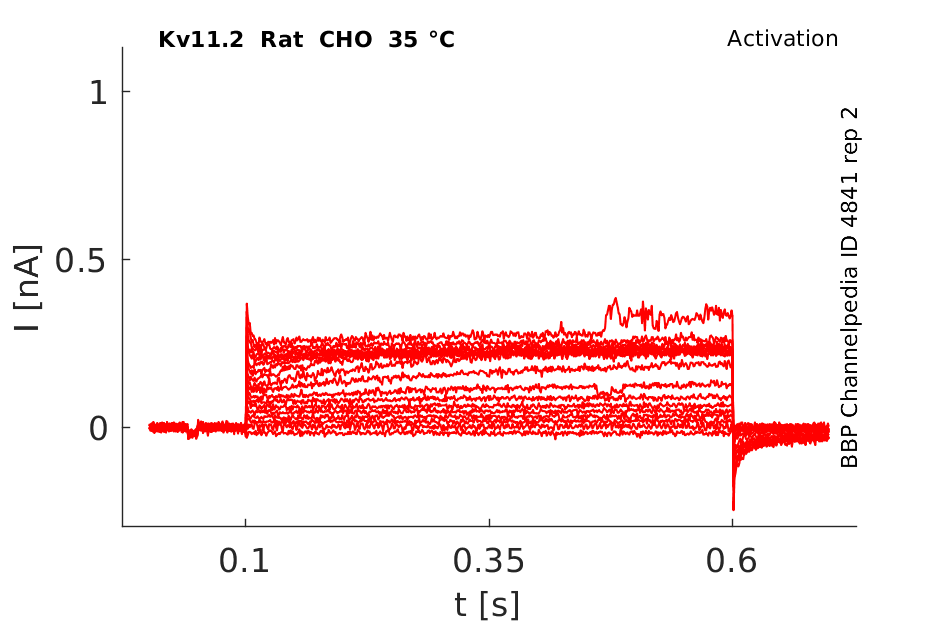
35 °Cshow 100 cells |
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_030779.4 | 3866 | |
| Mouse | NM_001037712.2 | 4438 | |
| Rat | NM_053937.1 | 3382 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv11.2 Structure
Methodology for visual representation of structure available here
Schematic diagram of Kv11.2
Schematic diagram of a Kv11 subunit containing six alpha-helical transmembrane segments S1–S6 and the pore P. The locations of the N-terminal PAS- domain and the C-terminal cyclic nucleotide binding domain (CNBD) are highlighted in dark and light blue, respectively. [796]
The ether-a-go-go gene K+ channel alpha-subunits consist of six membrane-spanning domains (S1–S6). A Per-Arnt-Sim (PAS) domain is located at the N-terminus and a cyclic nucleotide-binding domain (cNBD) in the C-terminus. This, and the tetrameric structure of a K+ channel can be seen in fig1 of Bauer [778]. The pore region P and S6 may form the inner core of the channel, S1–S5 the outer parts of the channel with S4 as the voltage sensor. [778]
Drug Binding Site to hERG channel

Kv11.2 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Kinetics of human fetal Kv11.2 in X. oocytes

Like eag1 currents, eag2 currents exhibit a pronounced Cole-Moore shift and they are blocked by low concentrations of cytosolic Ca2+ [327], [800]. However, the activation threshold of eag2 channels is much more negative (−100 mV) than that of rat eag1 channels (−50 mV)[800], and eag2 channels exhibit only a weak potential dependence. [778]
Rat Kv11.2 (erg2) Expressed in CHO cells

Activation curves of Erg1a, Erg1b, Erg2 (Kv11.2) and Erg3 channels
Voltage dependence of Erg current activation and inactivation in Purkinje cells compared to currents of Erg1a, Erg1b, Erg2 and Erg3 channels heterologously expresssed in HEK293 cells. Activation curves of erg1b and erg2 were shifted to more positive potentials by ∼20 and >40 mV, respectively, compared to erg1a and erg3 currents.[1737]
Expression in CNS
Kv11.2 and Kv11.3 are thought, contrary to Kv11.1, to be found exclusively in the nervous system [790]. However, recently, Kv11.2 and Kv11.3 transcripts were shown in rat pancreatic islets [798]. In rodent brain, the three channel subtypes display a widespread expression pattern although one study did detect Kv11.2 almost exclusively in the olfactory bulb [327]
Herg channel expression in Brain
All three Kv11 channels are expressed in the olfactory bulb, and erg1 and erg3 are co-expressed in the reticular thalamic nucleus, cerebral cortex, cerebellum and hippocampus [327]. Single cell RT-PCR experiments have shown that the erg subunits can be expressed in different combinations in individual rat lactotroph cells [781]. In addition, transcripts for more than one erg subunit have been detected in various cell lines: NG108-15 (neuroblastoma, erg1–3, [793]), PC12 (sympathetic ganglia neuron, erg1 and erg2, [327]), MMQ (lactotroph, erg1–3, [794]) and GH3/B6 (somatomammotroph, erg1 and erg2, [789]).
Expression in Rat
Kv11.2 and Kv11.3 are thought, contrary to Kv11.1, to be found exclusively in the nervous system [790]. However Kv11.2 and Kv11.3 transcripts were shown in rat pancreatic islets [798]. In rodent brain, the three channel subtypes display a widespread expression pattern [799], [326], [327]. although one study [327] did detect Kv11.2 almost exclusively in the olfactory bulb. (From [796])
Mouse Retina
By real-time PCR experiments we were able to show that Kv11.1 and Kv11.2 are expressed in a comparable level in the mouse retina while Kv11.3 expression was significantly lower [1734]
Pre vertebral Sympathetic Ganglia
The erg2 gene is expressed abundantly in the two prevertebral sympathetic ganglia examined: the celiac ganglia (CG) and the superior mesenteric ganglia (SMG) [790]
Tissue distribution of herg2

Kv11.2 Distribution in Neuronal Retina
In contrast to this postsynaptic distribution of Kv11.1 subunits in the OPL, Kv11.2 seemed to be expressed presynaptically in this layer (see Fig. 2B). The additional occurrence of Kv11.2 in dense packages in the IPL indicates the expression of Kv11.2 subunits presynaptically in ribbon synapses of both, photoreceptors and bipolar cells. As these synapses are glutamatergic, we performed double-labeling experiments with Kv11.2- and vGluT1-specific antibodies [1734]
Retina
Kv11.2 channels are expressed presynaptically in glutamatergic synapses of the retina, suggesting their contribution to the synaptic release of glutamate in photoreceptors and bipolar cells. The axons of OFF bipolar cells terminate in the outer half of the IPL, whereas those of ON and rod bipolar cells terminate in the inner half of the IPL. Accordingly, the co-localization of Kv11.2 and vGluT1 throughout the IPL strongly suggests that Kv11.2 subunits are expressed in all types of bipolar cells, but not in amacrine cells which is supported by the absence of Kv11.2 and GAD65 co-staining [1734]
Cytosolic Ca2+
Like eag1 currents, eag2 currents are blocked by low concentrations of cytosolic Ca2+ [327], [800].
Erg1 and Erg3
In the central nervous system of mammals, two other ERG channel genes are expressed (ERG2 and ERG3)11, and here heteromultimeric channel complexes of all three subunits can be formed [792]
RPR regulates hERG1 and rERG2 differentially
RPR260243 (RPR) induces voltage-dependent slowing of hERG1 deactivation. A study using site-directed mutagenesis proposed the C-linker domain as key component of slow deactivation in ERG channels and found that residues in the C-linker and the adjacent cyclic nucleotide-binding homology domains are sufficient to explain the different sensitivities of hERG1 and rERG2 to RPR.[2091]
References
Wimmers S
et al.
Biophysical properties of heteromultimeric erg K+ channels.
Pflugers Arch.,
2002
Dec
, 445 (423-30).
Papa M
et al.
Expression pattern of the ether-a-gogo-related (ERG) K+ channel-encoding genes ERG1, ERG2, and ERG3 in the adult rat central nervous system.
J. Comp. Neurol.,
2003
Nov
3
, 466 (119-35).
Saganich MJ
et al.
Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain.
J. Neurosci.,
2001
Jul
1
, 21 (4609-24).
Akbarali HI
et al.
Role of HERG-like K(+) currents in opossum esophageal circular smooth muscle.
Am. J. Physiol.,
1999
Dec
, 277 (C1284-90).
Bauer CK
et al.
A functional role of the erg-like inward-rectifying K+ current in prolactin secretion from rat lactotrophs.
Mol. Cell. Endocrinol.,
1999
Feb
25
, 148 (37-45).
Sanguinetti MC
et al.
A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel.
Cell,
1995
Apr
21
, 81 (299-307).
Overholt JL
et al.
HERG-Like potassium current regulates the resting membrane potential in glomus cells of the rabbit carotid body.
J. Neurophysiol.,
2000
Mar
, 83 (1150-7).
Bauer CK
et al.
RERG is a molecular correlate of the inward-rectifying K current in clonal rat pituitary cells.
Recept. Channels,
1998
, 6 (19-29).
Shi W
et al.
Identification of two nervous system-specific members of the erg potassium channel gene family.
J. Neurosci.,
1997
Dec
15
, 17 (9423-32).
Warmke JW
et al.
A family of potassium channel genes related to eag in Drosophila and mammals.
Proc. Natl. Acad. Sci. U.S.A.,
1994
Apr
12
, 91 (3438-42).
Wimmers S
et al.
Erg1, erg2 and erg3 K channel subunits are able to form heteromultimers.
Pflugers Arch.,
2001
Jan
, 441 (450-5).
Meves H
et al.
Separation of M-like current and ERG current in NG108-15 cells.
Br. J. Pharmacol.,
1999
Jul
, 127 (1213-23).
Lecchi M
et al.
Isolation of a long-lasting eag-related gene-type K+ current in MMQ lactotrophs and its accommodating role during slow firing and prolactin release.
J. Neurosci.,
2002
May
1
, 22 (3414-25).
Wulfsen I
et al.
Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary.
J. Neuroendocrinol.,
2000
Mar
, 12 (263-72).
Einarsen K
et al.
Functional properties of human neuronal Kv11 channels.
Pflugers Arch.,
2009
Aug
, 458 (689-700).
Chiesa N
et al.
A novel role for HERG K+ channels: spike-frequency adaptation.
J. Physiol. (Lond.),
1997
Jun
1
, 501 ( Pt 2) (313-8).
Mühlbauer E
et al.
Circadian changes of ether-a-go-go-related-gene (Erg) potassium channel transcripts in the rat pancreas and beta-cell.
Cell. Mol. Life Sci.,
2007
Mar
, 64 (768-80).
Guasti L
et al.
Expression pattern of the ether-a-go-go-related (ERG) family proteins in the adult mouse central nervous system: evidence for coassembly of different subunits.
J. Comp. Neurol.,
2005
Oct
17
, 491 (157-74).
Ludwig J
et al.
Cloning and functional expression of rat eag2, a new member of the ether-à-go-go family of potassium channels and comparison of its distribution with that of eag1.
Mol. Cell. Neurosci.,
2000
Jul
, 16 (59-70).
Cordeiro S
et al.
Expression pattern of Kv11 (Ether à-go-go-related gene; erg) K+ channels in the mouse retina.
PLoS ONE,
2011
, 6 (e29490).
Niculescu D
et al.
Erg potassium currents of neonatal mouse Purkinje cells exhibit fast gating kinetics and are inhibited by mGluR1 activation.
J. Neurosci.,
2013
Oct
16
, 33 (16729-40).
Sanguinetti MC
et al.
hERG potassium channels and cardiac arrhythmia.
Nature,
2006
Mar
23
, 440 (463-9).
Gardner A
et al.
C-Linker Accounts for Differential Sensitivity of ERG1 and ERG2 K+ Channels to RPR260243-Induced Slow Deactivation.
Mol. Pharmacol.,
2015
Jul
, 88 (19-28).
Contributors: Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/36/ , accessed on 2026 Feb 26