Kv4.3
Description: potassium voltage-gated channel, Shal-related subfamily, member 3 Gene: Kcnd3 Alias: Kv4.3, kcnd3, Kncd3
Kv4.3, encoded by the gene KCND3, is a member of the potassium voltage-gated channel subfamily D. Kv4.3 regulate repolarization of cardiac action potentials, firing frequency in neurons and dendritic excitability. [298]
Experimental data
Rat Kv4.3 gene in CHO host cells datasheet |
||
|
Click for details 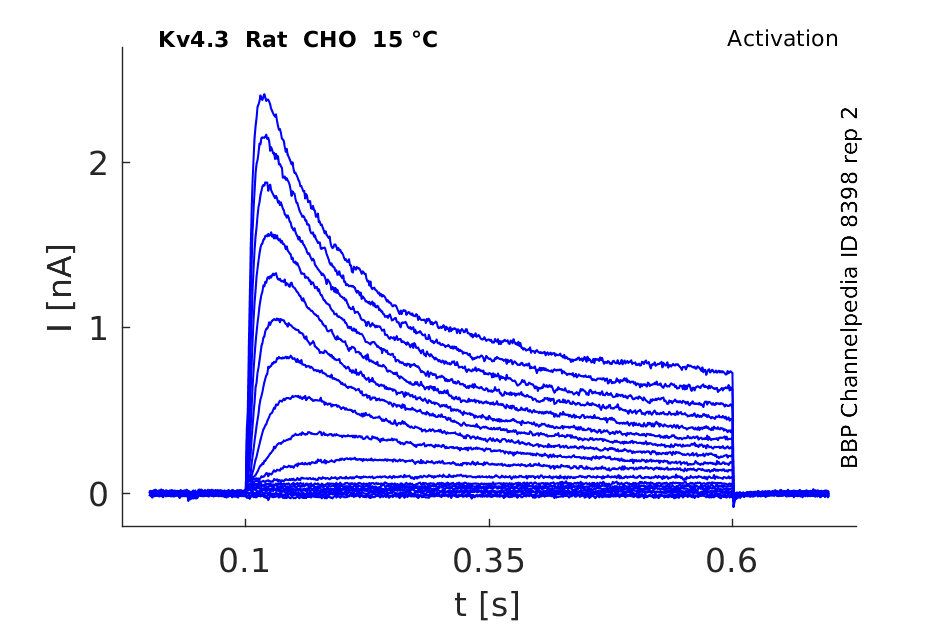
15 °Cshow 67 cells |
Click for details 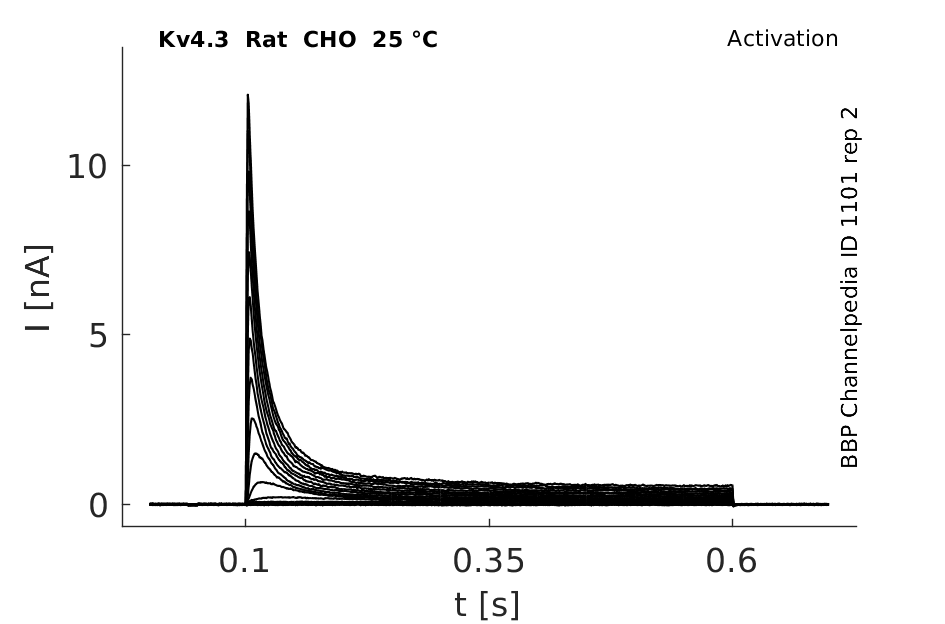
25 °Cshow 92 cells |
Click for details 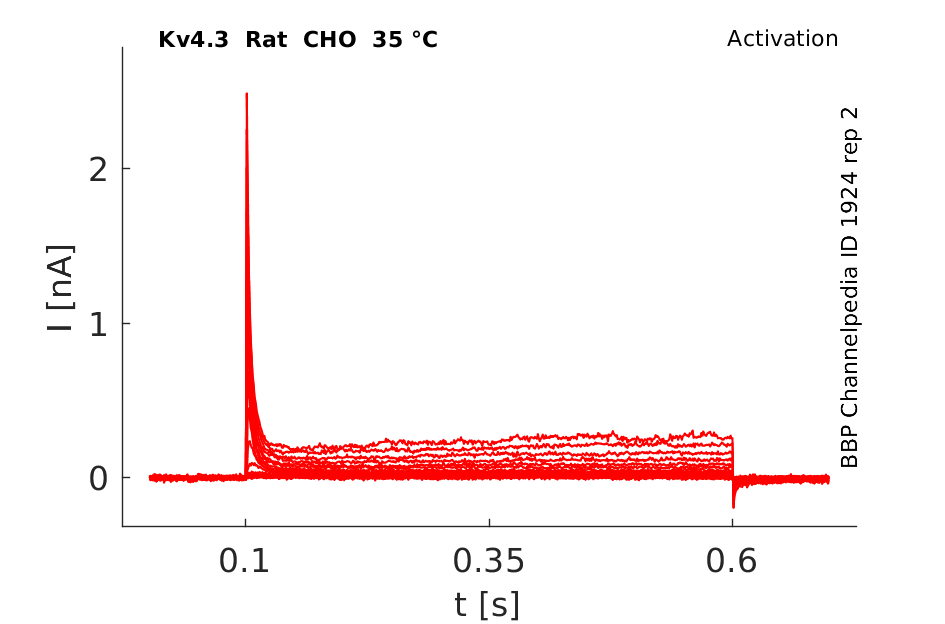
35 °Cshow 84 cells |
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_004980.5 | 7882 | |
| Mouse | NM_001039347.2 | 7609 | |
| Rat | NM_001270962.1 | 6818 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv4.3 Structure
Methodology for visual representation of structure available here
Altering Residues
Changing arginine residues in the S4 domain of Kv4.3 with alanine altered activation and deactivation processes as well as closed-state inactivation and recovery - consistent with general predictions of the Kv1 channel model. [297]
The absence of the basic residue R1 in Kv4.3 can only partially account for differences in the effective voltage sensitivity of gating between Shaker (Kv1) - in which R1 is found - and Kv4.3. [298]
Kv4.3 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Single Channel Conductance
4ps [467]
Kv4.3 Kinetics vary with Temperature
Our data demonstrated that the biophysical characteristics of Kv4.3/KChIP2 channels are strongly dependent on temperature. The time courses of activation, inactivation and recovery from inactivation were accelerated with increased temperature. KCNE1 did not significantly affect the current kinetics of Kv4.3/KChIP2 channels at 23 °C or 37 °C. In contrast to KCNE1, KCNE2 significantly slowed the time courses of activation, inactivation and recovery from inactivation of Kv4.3/KChIP2 channels at physiological temperature [1556]
KV4.3 Inactivation
The steady-state inactivation of Kv4.3 was examined using a standard two-pulse protocol. Representative Kv4.3 currents are shown in Fig. 6a. The steady-state inactivation curve was also shifted in the hyperpolarizing direction after treatment with mosapride. The half-inactivation potential (V1/2) and the slope factor (k) of the steady-state inactivation curve changed from −58.1±1.0 and 5.2±0.1 mV under control conditions to −62.9±1.8 and 5.3±0.2 mV (n=10, P<0.05), respectively.
Kv4.3 Kinetics
Under control conditions, the time course of Kv4.3 inactiva- tion at +40 mV can be described as a biexponential process with a fast time constant of 21.1±2.4 ms and a slow time constant of 143.4 ± 19.7 ms (n = 8). The fast and slow components of inactivation represented 83.9 ± 2.5 and 16.1 ± 2.5 % (n = 8) of total inactivation, respectively.
Markov Model Kv4.3
Our model for activation of the Kv4.3 channel contains only five closed states, with four voltage-dependent and one voltage-independent transitions. We investigated the time course of activation development and found that the best fit is achieved with the exponential function C[1 − exp(−t/τact)]n, where n = 4 or n = 5, but the differences between the fits were minor. Therefore, we used a model with four voltage-dependent steps to analyse Kv4.3 channel activation [449]
Markov Model (transition rates voltage AND TEMPERATURE dependent)
This model consists of four closed states (C0–C3), four closed-inactivated states (CI0–CI3), one open state (O) and one open-inactivated state (OI). Expanding the model, the transition rates αa and βa are designed here as voltage- as well as temperature-dependent, transition rates αi and βi are temperature-dependent only. Scaling factors (f1–f4; b1–b4) permit coupling of inactivation to activation as reported [1556]
Hodgkin and Huxley Model of Activation
A classical Hodgkin–Huxley model of activation should show a smooth bell-shaped voltage-dependent transition between τact and τdeact. As shown in the figure there is a steep transition between the τact and the τdeact over a narrow voltage range between −60 and −40 mV at 2 mm[K+]o, which was more marked at high [K+]o [449]
Hodgkin and Huxley Model of Open State Inactivation
Activation kinetics obtained from simulated data that did and did not contain a fast open-inactivated state using a Hodgkin–Huxley-like activation equation C[1 − exp(−t/τact)]4 [449]
Expression in the Brain
While in rat brain Kv4.2 and Kv4.3 are prevalent, all Kv4 proteins are clearly present in human brain. Neurons in the substantia nigra pars compact, the restrosplenial cortex, the superior colliculus, the raphe, and the amygdala express mainly Kv4.3. In the olfactory bulb Kv4.3 is highly expressed in periglomerular cells and in CA2-CA3 pyramidal cells and in granule cells of the dentate gyrus [466]
Expressed in Several Tissues
Finally, the mRNAs for Kv4.1 and Kv4.3 have been found in a variety of other tissue including skeletal muscle, spleen, thymus, bone marrow, liver, pancreas, prostrate, and kidney tissue [466]
Kv4.3 is abundant in rat adult brain and heart tissues.[328]
Only Kv4.3L (long version), but not Kv4.1, Kv4.2, Kv1.4, or Kv3.4, is expressed in single dopaminergic substantia nigra neurons [467]
Co-localization with KChIP1
KChIP1 co-localizes with Kv4.3 in hippocampal interneurons, cerebellar granule cells and synaptic glomeruli within the cerebellar granule cell layer. Co-immunoprecipitation analyses confirmed that the KChIPs are tightly associated with Kv4 alpha-subunits in brain potassium channel complexes [1195]
Distribution of Kv4.3 and 4.2 in Mouse Neuron
In all layers, clusters of Kv4.2 and Kv4.3 immunoreactivity are evident in the membranes of the somata, dendrites, and spines of pyramidal cells and GABAergic interneurons. Electron microscopic analyses revealed that Kv4.2 and Kv4.3 clusters in pyramidal cells and interneurons are excluded from putative excitatory synapses, whereas postsynaptic membranes at GABAergic synapses often contain Kv4.2 and Kv4.3. The presence of Kv4 channels at GABAergic synapses would be expected to weaken inhibition during dendritic depolarization by backpropagating action potentials. The extrasynaptic localization of Kv4 channels near excitatory synapses, in contrast, should stabilize synaptic excitation during dendritic depolarization. Thus, the synapse-specific distribution of Kv4 channels functions to optimize dendritic excitation and the association between presynaptic and postsynaptic activity [319]
Repolarizing action potential
Channels of the Kv4 (Shal-type) family generate rapidly activating and inactivating potassium currents in response to membrane depolarization, significantly modulating action potential repolarization and frequency-dependent electrical signaling. The current associated with Kv4 channels (IA) has thus been hypothesized to play important functional roles in diverse excitable cell types, including neuronal somatodendritic interactions [466] [467], long-term potentiation [468], pain plasticity [470], and excitation-contraction coupling in smooth and cardiac muscle [469].
Regulation of Electrophysiological Process
Kv4.3 channels have been implicated in the modulation of a wide range of electrophysiological processes in these cells: maintenance of membrane potential, regulation of the duration of action potential and the interspike interval, and attenuation of back-propagating action potentials [466]
Therapeutic targets of disease
Since alteration of Kv4.3 channel activities has resulted in several pathological conditions, these channels are considered to be a potential therapeutic target for diseases such as stroke, Alzheimer’s disease, arrhythmias, and cardiac hypertrophy [1555]
GI Smooth Muscle
Several lines of evidence have sug- gested that Kv4.3 currents may be one of the major molecular correlates of A-type Kv currents in many GI smooth muscles [467]
Kv4 regulates Spatial Memory
Significant differences in Kv4.2 and Kv4.3 mRNA levels were observed between conditioned and pseudoconditioned rats. Spatial learning performance was positively correlated with the levels of Kv4.2 and Kv4.3 mRNAs in several of these brain structures. Altogether our findings suggest that Kv4 channels could increase the signal-to-noise ratio during information acquisition, thereby allowing a better encoding of the memory trace [1557]
Atrial Fibrillation
Patients with paroxysmal or persistent AF have decreased Kv4.3 protein and mRNA levels, as well as reduced Ito current densities [1751]
KChIP
Calcium-binding proteins dubbed KChIPs favour surface expression and modulate inactivation gating of neuronal and cardiac A-type Kv4 channels. An allosteric kinetic model explaining kinetic changes that KChIP1 has on Kv4.1 and Kv4.3 channels can be found at [25].
At the cellular level, it has been shown that KChIP1 colocalizes with Kv4.3 in hippocampal interneurons and cerebellar granule cells. Based on the differential ability of KChIP2 and frequenin/NCS-1 to bind Kv4.3 proteins, coimmunoprecipitation analysis using KChIP2-frequenin chimeras shows that two EF-hand linkers (those between first and second EF hands and between third and fourth EF hands), and the C-terminal portion following the fourth EF hand are the determinants of KChIP association with Kv4.3 proteins [467]
Furthermore, mutations in the inner vestibule of Kv4.3 (V(399,401)I) significantly increased the sustained, noninactivating component and eliminated the ability of KChIP2b to accelerate slow phase of inactivation [467]
β-subunits
The effects of β-subunits specifically on Kv4.x channel function is still under investigation. While Kvβ1.2 does not affect Kv4.2 channel gating kinetics, it does confer sensitivity to redox modulation and hypoxia. Kv4.3 interacts with all three known β-subunits (β1, β2, and β3). Kvβ1 and 2 cause an increase in Kv4.3 channel current density, with no effect on channel gating, while β3 shifts steady-state inactivation and slows recovery from inactivation [466]
Kv4.3/KChIP2 channels without transmembrane β-subunits showed the strongest temperature sensitivity with considerably increased rates of activation and inactivation at 37 °C. KCNE2 significantly slowed the current kinetics at 37 °C compared to Kv4.3/KChIP2 channels, whereas KCNE1 did not influence the channel properties at both temperatures.
Rubidium Ions
When Rb+ is the current carrying ion in all Kv4 channels, both the development of deactivation and inactivation are slowed and the peak currents are increased.[27]
4-aminopyridine (4-AP)
4-AP blocks native Kv4 A-type K+ channels from cardiac tissue and cloned Kv4 K+ channels.[24] [26]. Kv4.2 also expresses suppression with a half-inhibition concentration at the low millimolar levels [467]
TEA
Kv4 currents and ISA have very similar pharmacological profiles. Both Kv4 current and ISA exhibit insensitivity to external TEA up to 20 mM [467]
DPPX
The association of DPPX with Kv4 channels increases current expression between 3 and 25 times due to increased trafficking, as determined by current recordings and immunohistochemistry. For Kv4.2 and Kv4.3, DPPX-S shifts the voltage dependence of activation and inactivation to more hyperpolarized potentials and significantly accelerates the time courses of activation, inactivation, and recovery from inactivation [467]
CaMKII
Although kinetic effects of CaMKII were not seen in studies on Kv4.2, exposure of rat Kv4.3 stably expressed in HEK cells to KN-93 or synthetic CaMKII inhibitor accelerates inactivation and slows recovery, whereas presence of autophosphorylated CaMKII slows inactivation and accelerates recovery. Thus, CaMKII phosphorylation may be more significant for regulating the levels of functional channels rather than the gating properties of the channels already present on the cell surface [467]
PKA
PKA activation does not modulate the gating of Kv4.2 when it is expressed alone. Likewise, application of phorbol 12-myristate 13-acetate (PMA) to oocytes expressing Kv4.2 or Kv4.3 currents produces a marked decrease in their peak amplitude in a dose dependent manner; however, like PKA there were no significant shifts in voltage dependence of activation. In studies of the expression Kv4.3 splice variants in mammalian L-cells, it was found that the long splice variant of Kv4.3 was selectively downregulated by PKC activation, due to a consensus PKC site contained in the alternative spliced exon ( [467]
Leptin
Leptin treatment induced an up-regulation of Kv4.2, Kv4.3 and KChIP2 subunits mRNA expression. Protein expression levels of Kv4.2, Kv4.3 and KChIP2 subunits were also increased by leptin. The electrophysiological study showed that leptin increases the fast transient outward potassium current amplitudes and densities shortening action potential duration [1553]
Mosapride and Cisapride
Mosapride and cisapride are gastroprokinetic agents with 5-hydroxytryptamine4 receptor agonist activity and have been widely used in the treatment of a variety of gastrointestinal disorders. Mosapride and cisapride inhibited Kv4.3 in a concentration-dependent manner with IC50 values of 15.2 and 9.8 μM, respectively. Mosapride accelerated the rate of inactivation and activation of Kv4.3 in a concentration-dependent manner and thereby decreased the time to peak [1554]
Ajmaline
Ajmaline is a class Ia anti-arrhythmic compound that is widely used for the diagnosis of Brugada syndrome and the acute treatment of atrial or ventricular tachycardia. When expressed in a mammalian cell line, ajmaline inhibits Kv4.3 with an IC50 of 2.66 μM [1558]
BMP4
Since the downregulation of Kv4.3 K(+) channels and the upregulation of BMP4 (bone morphogentic protein 4) simultaneously occur in pathological cardiac hypertrophy, we hypothesize that the up-regulated BMP4 would contribute to the downregulation of Kv4.3 K(+) channels in cardiac hypertrophy. We found that BMP4 treatment reduced Kv4.3 but not Kv4.2 and Kv1.4 K(+) channel protein expression [1559]
References
Jerng HH
et al.
Inactivation gating of Kv4 potassium channels: molecular interactions involving the inner vestibule of the pore.
J. Gen. Physiol.,
1999
May
, 113 (641-60).
Beck EJ
et al.
Remodelling inactivation gating of Kv4 channels by KChIP1, a small-molecular-weight calcium-binding protein.
J. Physiol. (Lond.),
2002
Feb
1
, 538 (691-706).
Song WJ
et al.
Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits.
J. Neurosci.,
1998
May
1
, 18 (3124-37).
Shahidullah M
et al.
The link between ion permeation and inactivation gating of Kv4 potassium channels.
Biophys. J.,
2003
Feb
, 84 (928-41).
Skerritt MR
et al.
Contribution of electrostatic and structural properties of Kv4.3 S4 arginine residues to the regulation of channel gating.
Biochim. Biophys. Acta,
2009
Feb
, 1788 (458-69).
Skerritt MR
et al.
Non-native R1 substitution in the s4 domain uniquely alters Kv4.3 channel gating.
PLoS ONE,
2008
, 3 (e3773).
Burkhalter A
et al.
Differential expression of I(A) channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses.
J. Neurosci.,
2006
Nov
22
, 26 (12274-82).
Serôdio P
et al.
Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain.
J. Neurophysiol.,
1998
Feb
, 79 (1081-91).
Serôdio P
et al.
Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain.
J. Neurophysiol.,
1996
May
, 75 (2174-9).
Wang S
et al.
Activation properties of Kv4.3 channels: time, voltage and [K+]o dependence.
J. Physiol. (Lond.),
2004
Jun
15
, 557 (705-17).
Birnbaum SG
et al.
Structure and function of Kv4-family transient potassium channels.
Physiol. Rev.,
2004
Jul
, 84 (803-33).
Jerng HH
et al.
Molecular physiology and modulation of somatodendritic A-type potassium channels.
Mol. Cell. Neurosci.,
2004
Dec
, 27 (343-69).
Sah R
et al.
Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to)).
J. Physiol. (Lond.),
2003
Jan
1
, 546 (5-18).
Hu HJ
et al.
The kv4.2 potassium channel subunit is required for pain plasticity.
Neuron,
2006
Apr
6
, 50 (89-100).
Gómez-Hurtado N
et al.
Prolonged leptin treatment increases transient outward K(+) current via upregulation of Kv4.2 and Kv4.3 channel subunits in adult rat ventricular myocytes.
Pflugers Arch.,
2013
Sep
18
, ().
Sung KW
et al.
Effect of mosapride on Kv4.3 potassium channels expressed in CHO cells.
Naunyn Schmiedebergs Arch. Pharmacol.,
2013
Oct
, 386 (905-16).
Wulff H
et al.
Voltage-gated potassium channels as therapeutic targets.
Nat Rev Drug Discov,
2009
Dec
, 8 (982-1001).
Radicke S
et al.
Accessory subunits alter the temperature sensitivity of Kv4.3 channel complexes.
J. Mol. Cell. Cardiol.,
2013
Jan
3
, ().
Truchet B
et al.
Kv4 potassium channels modulate hippocampal EPSP-spike potentiation and spatial memory in rats.
Learn. Mem.,
2012
, 19 (282-93).
Fischer F
et al.
Inhibition of cardiac Kv1.5 and Kv4.3 potassium channels by the class Ia anti-arrhythmic ajmaline: mode of action.
Naunyn Schmiedebergs Arch. Pharmacol.,
2013
Nov
, 386 (991-9).
Sun B
et al.
Bone morphogenetic protein-4 contributes to the down-regulation of Kv4.3 K+ channels in pathological cardiac hypertrophy.
Biochem. Biophys. Res. Commun.,
2013
Jul
12
, 436 (591-4).
Dixon JE
et al.
Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current.
Circ. Res.,
1996
Oct
, 79 (659-68).
Grammer JB
et al.
Molecular remodeling of Kv4.3 potassium channels in human atrial fibrillation.
J. Cardiovasc. Electrophysiol.,
2000
Jun
, 11 (626-33).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/17/ , accessed on 2026 Feb 25