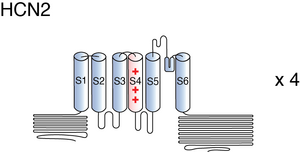
HCN2
Description: hyperpolarization activated cyclic nucleotide-gated potassium channel 2 Gene: Hcn2 Alias: HCN2, HAC1, BCNG2, ih2
HCN2, encoded by the gene hcn2, is a hyperpolarization-activated cyclic nucleotide-gated potassium channel. HCN2 is widely expressed throughout the body, though it is highest in specific areas of the central nervous system. It is involved in the generation of the I(h) current which controls neuron excitability.
Experimental data
Rat HCN2 gene in CHO host cells |
||
|
Click for details 
25 °Cshow 41 cells |
Click for details 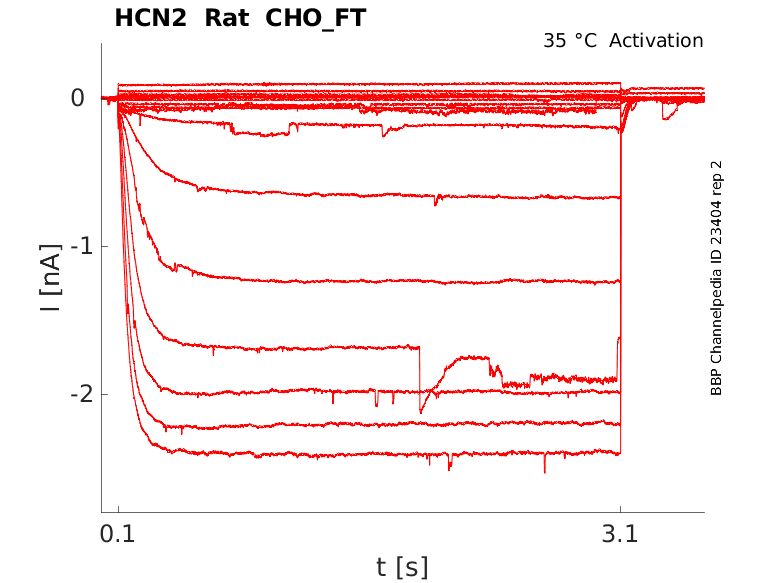
35 °Cshow 7 cells |
|
Mouse HCN2 gene in CHO host cells |
||
|
Click for details 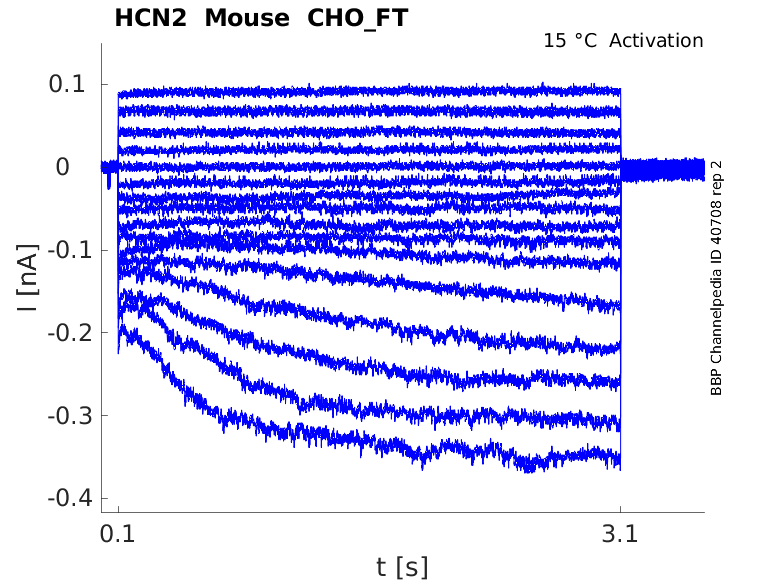
15 °Cshow 71 cells |
Click for details 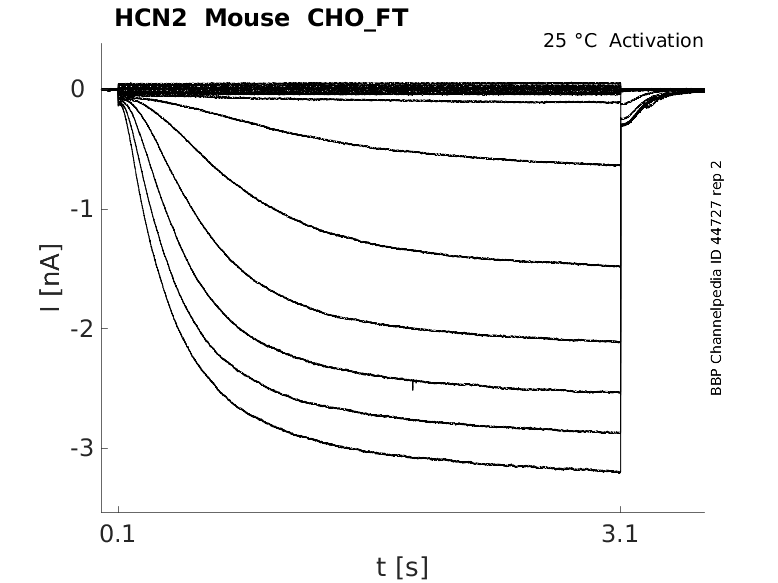
25 °Cshow 97 cells |
Click for details 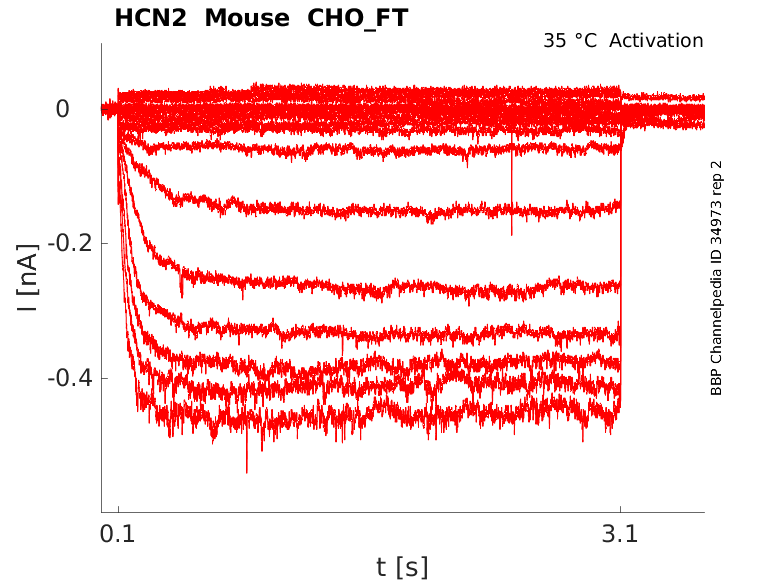
35 °Cshow 30 cells |
Human HCN2 gene in CHO host cells |
||
|
Click for details 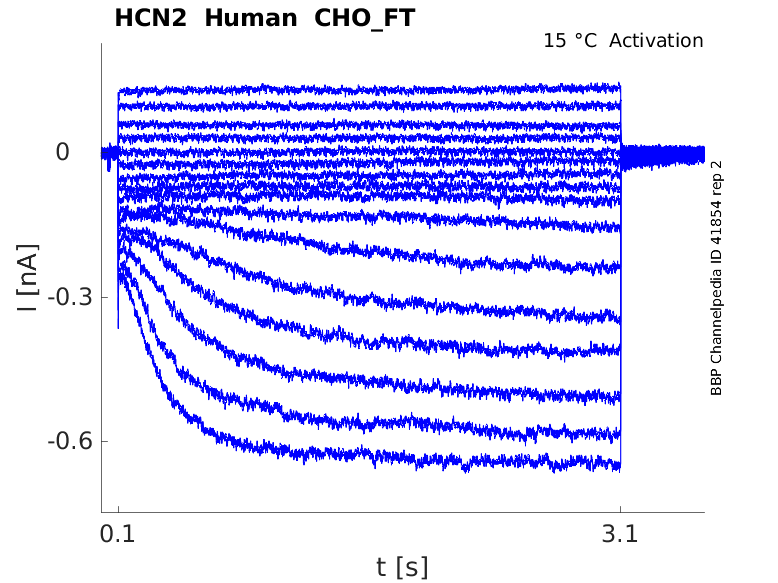
15 °Cshow 5 cells |
Click for details 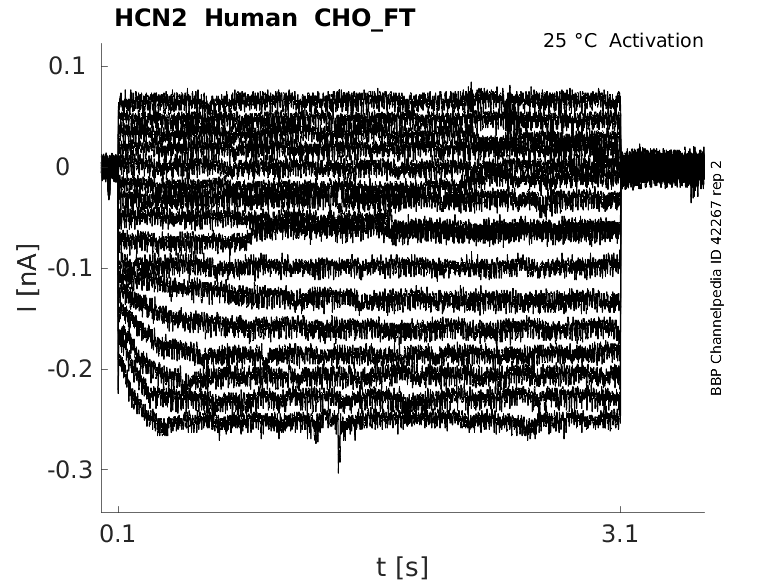
25 °Cshow 17 cells |
Click for details 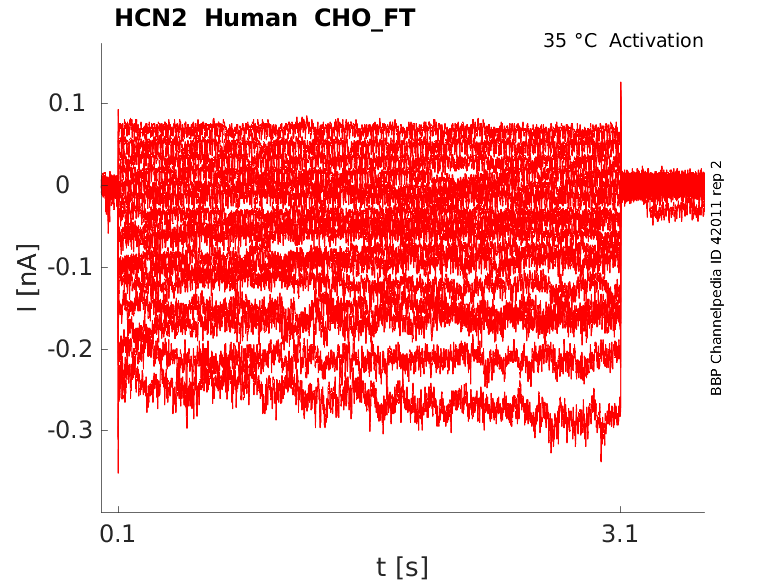
35 °Cshow 17 cells |
hcn2 is the coding gene for HCN2. In humans, hcn2 is located on chromosome 19p13.3 and is made up of 8 exons, all of which are coding.
To date, no transcript variants for hcn2 have been identified.
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_001194.4 | 3420 | |
| Mouse | NM_008226.2 | 3195 | |
| Rat | NM_053684.1 | 2633 |
The human HCN2 protein is composed of 889 amino acids (aa) and has a molecular weight of ~97 Kda.
To date, no isoforms resulting from alternative splicing have been identified.
Isoforms
Like most mammalian proteins, HCN2 is subject to a series of post translational modifications (PTM).
HCN2 undergoes N-linked glycosylation, which plays a pivotal role in facilitating its cell surface expression. In experimental settings, blocking of glycosylation by tunicamycin or removal of key glycosylation sites via mutation experiments lead to lack of insertion of HCN2 in the plasma membrane, resulting in no whole-cell currents [2296].
HCN2 is subject to phosphorylation by various kinases, including serine/threonine kinases such as p38 mitogen-activated protein kinase, tyrosine kinases like Src, and cGMP-Dependent Protein Kinase II (cGKII). Src has been shown to interact with and phosphorylate HCN2 at the c-linker-CNBD, resulting in an acceleration of channel kinetics. Conversely, inhibiting Src-mediated phosphorylation leads to a deceleration of channel kinetics [2327] [457] cGKII, on the other hand, exerts an inhibitory effect on the voltage-dependent gating of HCN2 channels. It binds to the C-terminus of HCN2 channels, which ultimately leads to a hyperpolarizing shift in the voltage-dependent activation of these channels [2295]
SUMOylation is also involved in the regulation of HCN2. HCN2 SUMOylation was identified in mouse forebrain and SUMOylation of HCN2 in HEK cells increased current conductance and surface expression [2293]
HCN2 is subjected to S-palmitoylation with the N-terminal region containing five palmitoylation sites. Intriguingly, these post-translational modifications do not appear to significantly alter the fundamental physiological properties of HCN2, as evidenced by experiments conducted in HEK cells. Given this, it is plausible that S-palmitoylation in the N-terminal region of HCN channels might serve a regulatory function in channel formation or in the coassembly of HCN channels with auxiliary subunits.[2298]
Visual Representation of HCN2 Structure
Methodology for visual representation of structure available here
The structure HCN2 has not yet been resolved in its entirety. However, certain areas, particularly the C-terminal and CNBD have been characterized.
The HCN2 C-terminal region is composed of two domains: The C-linker domain, consisting of six α-helices, designated A′ to F′, which are separated by short loops, and the CNBD, which includes four α-helices (A, P, B, C) with a β-roll between the A- and B-helices. The β-roll comprises eight β-strands in a jelly-roll-like topology. [2294] Certain residues in the C-helix (R632, R635, I636, and K638) were shown to contribute to the higher selectivity of HCN2 channels for cAMP than for cGMP. However, it is not clear how these residues generate cAMP selectivity. [457]
The C-linker domain serves a dual role in the HCN channel structure. It both couples the ion channel gating machinery to the ligand-binding domain but also mediates most of the subunit–subunit contacts in the tetramer.
The structure of the C-terminal region and changes to this area are thought to be responsible for the impact of cAMP binding to HCN channels. In the absence of cAMP, the C-terminal region is thought to exert a tonic inhibition on the pore when the C-linker and CNBD domains are in a non-tetrameric form [2303]. When the cAMP concentration increases, the C-linker/CNBD region tetramerizes and releases the inhibition from the pore gate. This event results in the positive shift of 16–20 mV in the activation curve of HCN2 and HCN4. [2304]
HCN2 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
HCN2 ranks as the second-fastest member within the HCN channel family, second to only to HCN1. When comparing the activation properties, HCN2 exhibits activation at more negative potentials (~20 mV) than HCN1. Importantly, at any given voltage, the activation kinetics of HCN2 has been demonstrated to be approximately 15-fold slower than those of HCN1.[2300]
Like other HCN channels, HCN2 is thought to also undergo mode shifting. However, effects of voltage hysteresis are less pronounced but though still have effect on deactivation kinetics [457]
Single channel unitary conductance
Single channel unitary conductance is determined experimentally. For HCN2, there have been recordings of single channel unitary conductance values with some degree of variance between experiments:
- 1.7 pS [2293]
Model
Model HCN2 (ID=10)
| Animal | Mouse | |
| CellType | Dorsal root ganglion | |
| Age | 21 Days | |
| Temperature | 0.0°C | |
| Reversal | -45.0 mV | |
| Ion | Hcn + | |
| Ligand ion | ||
| Reference | [57] S Moosmang et. al; Eur. J. Biochem. 2001 Mar | |
| mpower | 1.0 | |
| m Inf | 1.0000/(1+exp((v- -99)/6.2)) | |
| m Tau | 184.0000 | |
Cellular and tissue
HCN2 is distributed across the peripheral (PNS) and central nervous system (CNS), though is more predominant in the latter. [323]
HCN2 is expressed at very high levels nearly ubiquitously in the cortical and subcortical regions of the brain, with the strongest levels measured in the olfactory bulb, hippocampus, thalamus, and brainstem [338]. Other abundant areas include: the mammillary bodies, pontine nucleus, ventral cochlear nucleus, and nucleus of the trapezoid body [337]. HCN2 is also present in CNS non excitable cells, namely glial cells such as oligodendrocytes [2292] [2306] [2328].
In the PNS, HCN2 was identified in areas such as the dorsal root ganglion, the nodose neurons, the heart, the retina, and a number of small sensory neurons [2311] [457] [2306] [2329].
For a more detailed map immunohistochemical localisation of HCN2, please consult the following resource [323]
Developmental
There is evidence that HCN2 expression is developmentally regulated. In the rat thalamocortical relay neurons, quantitative analysis revealed that, along with HCN1 and HCN4, HCN2 expression levels increase over development, reaching its highest levels in adulthood. [2314] HCN2 immunoreactivity was detected as early as P1 and increased steadily up to P20. [2313] This developmental increase is accompanied with an increase in I(h) current density as a result from increased mRNA expression. [2314]
Developmental changes affect not only the expression pattern of HCN2 but also its distribution in the cell. Indeed, HCN2 expression starts in the soma before migrating to the dendrites over a period of about two weeks during rat development. These changes in developmental distribution are influenced by other factors, such as accessory proteins (TRIP8b). [2295]
The subcellular localization of HCN channels is neuron-type-specific. It is therefore difficult to generalize and pinpoint the exact cellular locations of these channels.
In pyramidal cells, HCN2 can be found in the distal dendrites. This corroborates with electrophysiological studies that show that HCN I(h) current is greatest in the distal dendrites of cortical and hippocampal pyramidal neurons [2307]. In the interneurons of the medial septum, hippocampus, and cerebellum, HCN2, along with other HCN channels, are expressed at somatic and axonal regions [2295].
HCN2, like HCN4, is also present in the receptor terminals of both myelinated and unmyelinated fibers [2311].
HCN2 is responsible for a number of functions throughout the body
Neuronal activity
HCN2 plays an important role in controlling neuronal excitability. In the central nervous system, HCN2 channels contribute to maintaining the resting membrane potential and controlling the frequency and timing of action potentials. They are particularly important in certain types of neurons involved in rhythmic activities like the generation of respiratory patterns, as well as in regulating the excitability of sensory neurons. In the thalamus, HCN2 is the major component responsible for thalamocortical I(h). Deletion of HCN2 showed a ~80% reduction of thalamocortical I(h), resulting in a 12 mV hyperpolarizing shift in the resting membrane potential. Furthermore, the thalamocortical neurons of HCN2-deficient mice displayed a higher susceptibility to fire in the burst mode in response to excitatory inputs compared to wild-type neurons [457] [2330].
Pacemaker activity
As previously mentioned, HCN2 is expressed in the heart, particularly the sinoatrial node, an area responsible for pacemaking. HCN2 makes up ∼20% of SA node I(h) in mice and may serve as a complementary channel to HCN4. Genetic mouse studies indicate that the two components of SA node I(h), carried by HCN4 and HCN2, are required for maintaining a stable cardiac rhythm. However, neither HCN2 nor HCN4 seem to be needed for principal pacemaking and for the autonomous rate regulation in the adult heart [457].
Sensory processing, nociception and pain disorders
As HCN2 is expressed in a number of sensory neurons, the channel is involved in multiple sensing modalities.
HCN2 channels are involved in shaping the response to sensory stimuli. HCN2’s contribution to proper sensory processing is particularly highlighted within the context of nociception and pain disorders.
Indeed, case studies have shown that patients with specific mutations in HCN2 were shown to have heightened sensitivity to temperature. Functional investigations showed that the mutations lead to an increased I(h) availability at high temperatures (38°C), which may contribute to hyperthermia-induced neuronal hyperexcitability [2331]. HCN2 was also identified as a driver for pain in mouse models of diabetic neuropathy [2332]. HCN2 channel activation shifted the resting membrane potential to depolarized values and increased action potential firing in neurons, contributing to acute neuronal hypersensitivity. [2293]
Furthermore, genetic deletion or pharmacological block of HCN2 were shown to abolish hyperalgesia in inflammatory pain and neuropathic pain experiments [2331] [2329].
These studies provide compelling evidence supporting the hypothesis that HCN2 plays a crucial role in the generation and maintenance of pain. Mutations in HCN2 have been identified as responsible for the dysregulation of the channel, contributing to the development of heightened inflammatory and neuropathic pain. [2318] [2333]
Channelopathies
On top on pain, mutations to HCN2 have been associated with several other pathologies including:
- Epilepsy [2319] [2334]
- Absence seizures [2335]
- Affective disorders [2336] [2306]
- Mice with reduced HCN2 expression subjected to chronic stress displayed a reduction in tonic firing rates in specific brain regions, which was correlated with depressive behavior. Conversely, increasing the expression or overexpressing HCN2 effectively alleviated this depressive-like phenotype. These findings strongly imply that augmenting HCN2 channel activity holds promise as a potential strategy for the development of a novel class of antidepressant medications.
HCN2, like other channels, is regulated by various auxiliary proteins and secondary messengers. These regulatory proteins play important roles in the development, localization, and expression of the channel protein.
Heteromeric channels
There is evidence that certain HCN channels, coexpressed in the same regions, coassemble to generate heteromultimeric channels. For example, HCN2 can be found in conjunction with HCN1 in certain areas, leading to the formation of hybrids consisting of both HCN2 and HCN1 subunits. These heteromultimeric isoforms of HCN channels exhibit I(h) currents with activation kinetics and voltage dependence that tend to fall in between those of HCN1 and HCN2 homomers. These heteromers also display a relatively large shift by cAMP (+14 mV) [1697] [2296].
Cyclic nucleotides
HCN2 is highly sensitive to cyclic adenosine monophosphate (cAMP), which binds to the protein via the CNBD, located in the intracellular C-terminal. Interaction with cAMP shifts the V1/2 of HCN2 by +17.3 ± 1.0 mV and accelerating activation by 3.5 fold. [2300] The mechanisms of this modulation are further explained in Structure and [here] (link to HCN general page & structure)
Other cyclic nucleotides have also been shown to interact and modify HCN2 behavior:
- Pyrimidine cyclic nucleotide cCMP: cCMP shifts the activation curves of, HCN2 and HCN4, to more depolarized voltages, speeds up activation, and slows down deactivation kinetics of these channels. [2302]
- Uridine 3′,5′-cyclic monophosphate (cUMP), purine 3′,5′-cyclic monophosphate (cPMP), and 2-amino-cPMP shift the activation curve to more positive potentials and increase channel conductance.[2293]
- Inosine 3′,5′-cyclic monophosphate (cIMP) & cCMP, are weak activators HCN2 [2293]
- Cyclic guanosine monophosphate (cGMP) slightly accelerates activation kinetics [56]
Other modulators
HCN2 is sensitive to Cl−. The regulation of HCN2 by Cl− is thought to be relevant in heart (patho-)physiology [457].
KCR1 is an inhibitory auxiliary subunit of HCN channels and plays a role in regulating cardiac automaticity. It reduces the current densities and alters the single-channel current parameters of HCN2 channels, resulting in reduced current size and a shift in I(h) activation to more negative potentials [457] [2293].
Murine HCN2 has been shown to be sensitive to pH. At acidic and alkaline pH, the midpoint potential of HCN2 activation is shifted by ∼10 mV to more hyperpolarized and depolarized potentials, respectively, compared with physiological pH. The modulation of HCN channels by intracellular protons may have an important physiological impact on the modulation of HCN channel activity in the brain, for example, for the regulation of thalamic oscillations and the respiratory frequency. [457]
Phosphatidylinositol-4,5- bisphosphate (PIP 2 ) is a phospholipids that acts as a ligand that allosterically opens HCN channels. Interaction of PIP2 and HCN2 results in a right-shift of the steady-state activation by ~20 mV [2337] [2338].
MiRP1 was found to interact with HCN2, leading to increased current densities and acceleration of its activation kinetics. However, MiRP1 did not affect voltage dependence of activation. [457]
HCN2 interacts with neuronal scaffold proteins tamalin, Mint2, and S-SCAM. The binding with the latter two are done via the COOH- terminal tail, whereas the former is through the PDZ-like binding domain. These proteins are thought to regulate HCN2 cell surface expression [457] [2339].
For further compounds interactions, please consult the following resource
References
Brandt MC
et al.
Effects of KCNE2 on HCN isoforms: distinct modulation of membrane expression and single channel properties.
Am. J. Physiol. Heart Circ. Physiol.,
2009
Jul
, 297 (H355-63).
Stieber J
et al.
Functional expression of the human HCN3 channel.
J. Biol. Chem.,
2005
Oct
14
, 280 (34635-43).
Moosmang S
et al.
Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues.
Eur. J. Biochem.,
2001
Mar
, 268 (1646-52).
Notomi T
et al.
Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain.
J. Comp. Neurol.,
2004
Apr
5
, 471 (241-76).
Monteggia LM
et al.
Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain.
Brain Res. Mol. Brain Res.,
2000
Sep
30
, 81 (129-39).
Moosmang S
et al.
Differential distribution of four hyperpolarization-activated cation channels in mouse brain.
Biol. Chem.,
1999 Jul-Aug
, 380 (975-80).
Biel M
et al.
Hyperpolarization-activated cation channels: a multi-gene family.
Rev. Physiol. Biochem. Pharmacol.,
1999
, 136 (165-81).
Giorgetti A
et al.
A homology model of the pore region of HCN channels.
Biophys. J.,
2005
Aug
, 89 (932-44).
Park K
et al.
HCN channel activity-dependent modulation of inhibitory synaptic transmission in the rat basolateral amygdala.
Biochem. Biophys. Res. Commun.,
2011
Jan
28
, 404 (952-7).
Biel M
et al.
Hyperpolarization-activated cation channels: from genes to function.
Physiol. Rev.,
2009
Jul
, 89 (847-85).
Meng QT
et al.
LOCAL ANESTHETIC INHIBITS HYPERPOLARIZATION ACTIVATED CATIONIC CURRENTS.
,
2011
Feb
8
, ().
Barbuti A
et al.
Localization of pacemaker channels in lipid rafts regulates channel kinetics.
Circ. Res.,
2004
May
28
, 94 (1325-31).
Benarroch EE
HCN channels: function and clinical implications.
Neurology,
2013
Jan
15
, 80 (304-10).
Reid CA
et al.
HCN channelopathies: pathophysiology in genetic epilepsy and therapeutic implications.
Br. J. Pharmacol.,
2012
Jan
, 165 (49-56).
Chen S
et al.
Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide.
J. Gen. Physiol.,
2001
May
, 117 (491-504).
Rivolta I
et al.
Cardiac and neuronal HCN channelopathies.
Pflugers Arch, 2020Jul, 472 (931-951).
Sartiani L
et al.
The Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels: from Biophysics to Pharmacology of a Unique Family of Ion Channels.
Pharmacol Rev, 2017Oct, 69 (354-395).
Zagotta WN
et al.
Structural basis for modulation and agonist specificity of HCN pacemaker channels.
Nature,
2003
Sep
11
, 425 (200-5).
He C
et al.
Neurophysiology of HCN channels: From cellular functions to multiple regulations.
Prog. Neurobiol.,
2014
Jan
, 112 (1-23).
Much B
et al.
Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels.
J. Biol. Chem.,
2003
Oct
31
, 278 (43781-6).
Itoh M
et al.
The hyperpolarization-activated cyclic nucleotide-gated (HCN) channels contain multiple S-palmitoylation sites.
J Physiol Sci, 2016May, 66 (241-8).
Wainger BJ
et al.
Molecular mechanism of cAMP modulation of HCN pacemaker channels.
Nature,
2001
Jun
14
, 411 (805-10).
Zong X
et al.
Regulation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel activity by cCMP.
J. Biol. Chem.,
2012
Aug
3
, 287 (26506-12).
Ulens C
et al.
Regulation of hyperpolarization-activated HCN channels by cAMP through a gating switch in binding domain symmetry.
Neuron,
2003
Dec
4
, 40 (959-70).
Lolicato M
et al.
Tetramerization dynamics of C-terminal domain underlies isoform-specific cAMP gating in hyperpolarization-activated cyclic nucleotide-gated channels.
J. Biol. Chem.,
2011
Dec
30
, 286 (44811-20).
Santoro B
et al.
Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels as Drug Targets for Neurological Disorders.
Annu Rev Pharmacol Toxicol, 2020Jan06, 60 (109-131).
Shah MM
Cortical HCN channels: function, trafficking and plasticity.
J. Physiol. (Lond.),
2014
Jul
1
, 592 (2711-9).
Doan TN
et al.
Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers.
J. Neurosci.,
2004
Mar
31
, 24 (3335-43).
Vasilyev DV
et al.
Postnatal development of the hyperpolarization-activated excitatory current Ih in mouse hippocampal pyramidal neurons.
J. Neurosci.,
2002
Oct
15
, 22 (8992-9004).
Kanyshkova T
et al.
Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations.
J. Neurosci.,
2009
Jul
8
, 29 (8847-57).
Lainez S
et al.
HCN3 ion channels: roles in sensory neuronal excitability and pain.
J Physiol, 2019Sep, 597 (4661-4675).
Kessi M
et al.
The Contribution of HCN Channelopathies in Different Epileptic Syndromes, Mechanisms, Modulators, and Potential Treatment Targets: A Systematic Review.
Front Mol Neurosci, 2022, 15 (807202).
DiFrancesco D
The role of the funny current in pacemaker activity.
Circ. Res.,
2010
Feb
19
, 106 (434-46).
Swire M
et al.
Oligodendrocyte HCN2 Channels Regulate Myelin Sheath Length.
J Neurosci, 2021Sep22, 41 (7954-7964).
Emery EC
et al.
HCN2 ion channels: an emerging role as the pacemakers of pain.
,
2012
May
19
, ().
Chan CS
et al.
HCN2 and HCN1 channels govern the regularity of autonomous pacemaking and synaptic resetting in globus pallidus neurons.
J. Neurosci.,
2004
Nov
3
, 24 (9921-32).
DiFrancesco JC
et al.
Dysfunctional HCN ion channels in neurological diseases.
Front Cell Neurosci,
2015
, 6 (174).
Tsantoulas C
et al.
Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy.
Sci Transl Med, 2017Sep27, 9 (eaam6072).
Weng X
et al.
Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Aδ-nociceptors.
Pain,
2012
Apr
, 153 (900-14).
DiFrancesco JC
et al.
Recessive loss-of-function mutation in the pacemaker HCN2 channel causing increased neuronal excitability in a patient with idiopathic generalized epilepsy.
J. Neurosci.,
2011
Nov
30
, 31 (17327-37).
Ludwig A
et al.
Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2.
EMBO J.,
2003
Jan
15
, 22 (216-24).
Cheng J
et al.
HCN2 Channels in Cholinergic Interneurons of Nucleus Accumbens Shell Regulate Depressive Behaviors.
Neuron, 2019Feb20, 101 (662-672.e5).
Zolles G
et al.
Pacemaking by HCN channels requires interaction with phosphoinositides.
Neuron,
2006
Dec
21
, 52 (1027-36).
Schmidpeter PAM
et al.
Anionic lipids unlock the gates of select ion channels in the pacemaker family.
Nat Struct Mol Biol, 2022Nov, 29 (1092-1100).
Kimura K
et al.
Hyperpolarization-activated, cyclic nucleotide-gated HCN2 cation channel forms a protein assembly with multiple neuronal scaffold proteins in distinct modes of protein-protein interaction.
Genes Cells,
2004
Jul
, 9 (631-40).
Contributors: Katherine Johnston, Rajnish Ranjan, Michael Schartner, Nitin Khanna
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/62/ , accessed on 2026 Feb 21