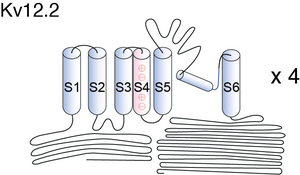
Kv12.2
Description: potassium voltage-gated channel, subfamily H (eag-related), member 3 Gene: Kcnh3 Alias: Kv12.2
Kv12.2, encoded by the gene KCNH3, is potassium voltage-gated channel subfamily H member 3 [606], [810]. It is also known as BEC1; ELK2; Kv12.2; KIAA1282.
Experimental data
Rat Kv12.2 gene in CHO host cells datasheet |
||
|
Click for details 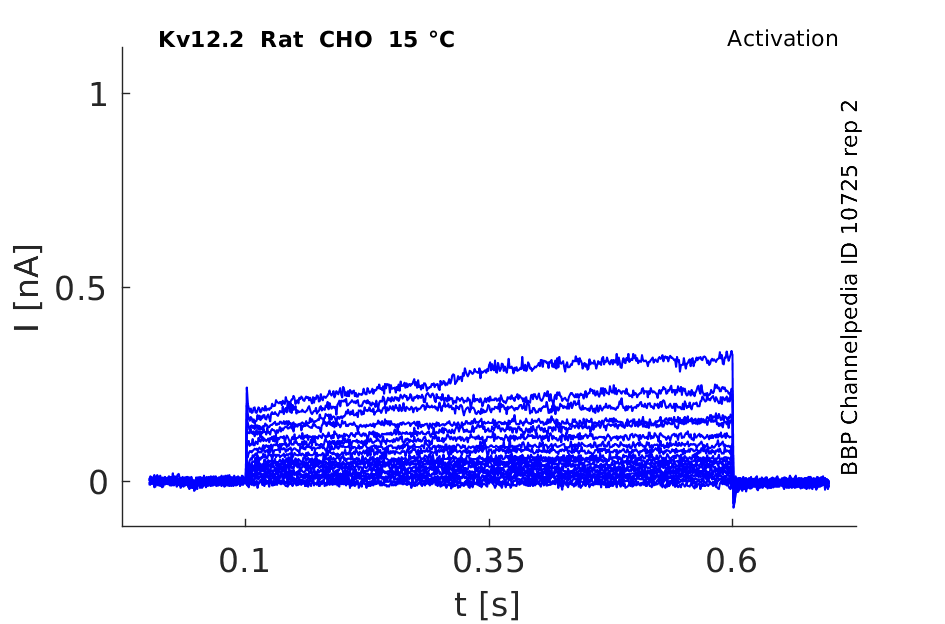
15 °Cshow 47 cells |
Click for details 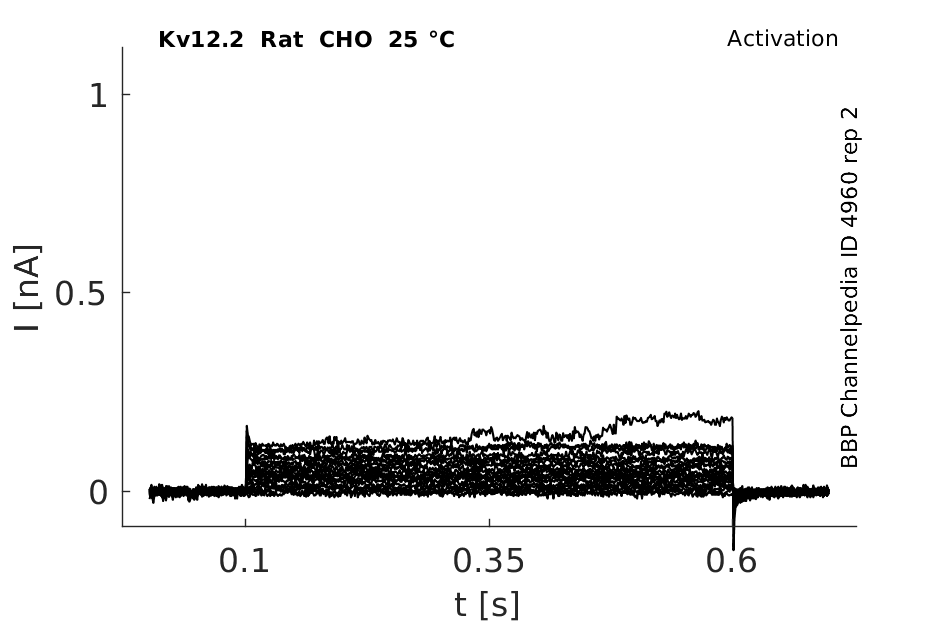
25 °Cshow 59 cells |
Click for details 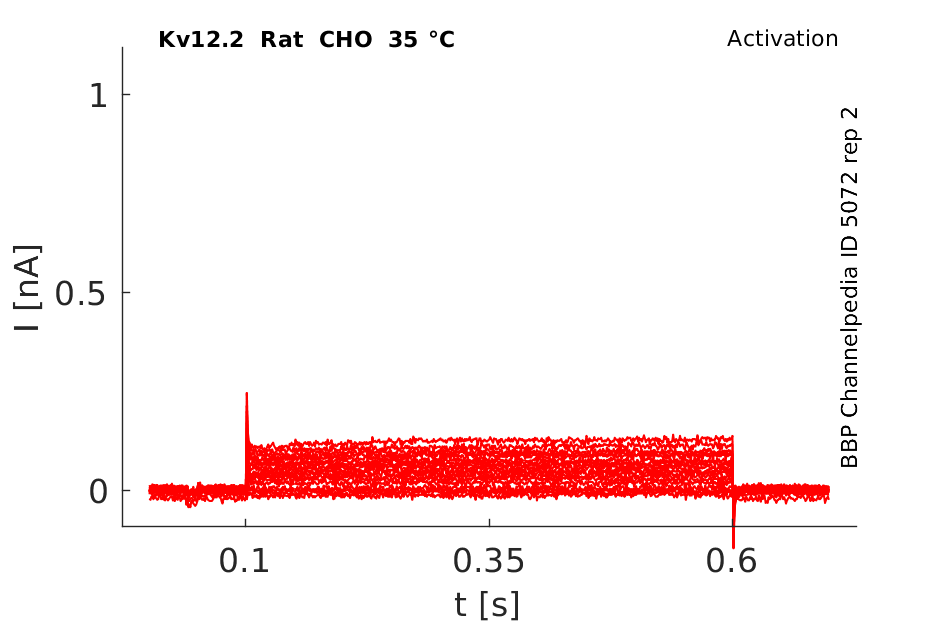
35 °Cshow 65 cells |
Kv12.2, also known as ether-a-go-go-like 2 (Elk2) or KCNH3, belongs to the ether-a-go-go (EAG) family, which comprises the ether-a-go-go (Eag, Kv10.x), ether-a-go-go-related gene (Erg, Kv11.x), and ether-a-go-go like (Elk, Kv12.x) subfamilies [812], [778].
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_012284.3 | 4023 | |
| Mouse | NM_010601.4 | 3967 | |
| Rat | NM_017108.1 | 3715 |
Unlike Kv5.x, Kv6.x, Kv8.x, and Kv9.x, which function as modifiers for other Kv channels [606], Kv12.2 can produce a functional channel on its own when heterologously expressed [809], [808], [813]. (From [802])
Isoforms
Glycosylation in CHO Cells
N-glycosylation effects the function of Kv12.2, in as much as that removal of sugar chains causes a depolarizing shift in the steady-state activation without a significant reduction in current amplitude. Unlike the previously reported shift for Shaker-type Kv channels, this shift does not appear to be due to negatively charged sialic acid residues in the sugar chains. Kv12.2 is N-glycosylated in Chinese hamster ovary (CHO) cells and in cultured neurons as well as in the mouse brain. Only glycosylated Kv12.2 channels show proper voltage dependence and are utilized in vivo. [802]
Visual Representation of Kv12.2 Structure
Methodology for visual representation of structure available here
There is a light oxygen voltage (LOV) and cyclic nucleotide binding (CNB) domain in the N and C terminus, respectively. [606]
Kv12.2 features the longest S5-P loop among all known mammalian Kv channels with the most N-linked glycosylation sites (three sites). [802]
Kv12.2 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Rat Kv12.2 channels very similar to HERG
RELK2 channels gave rise to slowly activating K+ currents. At more positive potentials, the evoked currents inactivated rapidly. Recovery from inactivation at negative potentials was reminiscent of that seen for HERG channels [809]
Mouse Kv12.2 in CHO cells display Current with EGFP
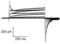
pH sensitivity of Kv12.1,Kv12.2 and Kv12.3
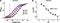
Kv12.2 was found in infant brain, lung (small cell carcinoma), eye (retinoblastoma), sciatic nerve, cortex, amygdala, hippocampus (mainly in CA1 and CA3 pyramidal cell body layers and in the granule cell layers of the dentate gyrus); in the striatal regions, including the putamen and caudate nucleus, lymphocytes, leukemias, and NG108-15 cell line. [327], [793], [810], [809], [811]. (Summary from [606])
Astrocytoma
ELK2 channels are very effective at dampening the neuronal excitability, but less so at producing adaptation of action potential firing frequency. In addition, we suggest experimental ways to recognize HELK2 currents in vivo and raise the issue of the possible function of these channels in astrocytoma [813]
Hyppocampal Hyperexcitability and Epilepsy
Human Kv12.2 may be implicated in epilepsy [814]. We show here that the voltage-gated K+ channel Kv12.2 is a potent regulator of excitability in hippocampal pyramidal neurons. Genetic deletion and pharmacologic block of Kv12.2 significantly reduced firing threshold in these neurons. Kv12.2−/− mice displayed signs of persistent neuronal hyperexcitability including frequent interictal spiking, spontaneous seizures and increased sensitivity to the chemoconvulsant pentylenetetrazol [803]
Cognitive Function
Disruption of the Ether-à-go-go K+ Channel Gene Kv12.2/KCNH3 Enhances Cognitive Function [1786]
Transcription of KCNH3
The transcription of KCNH3 the gene coding for Kv12.2 may be activated by the transcription factor FOXG1 in mature neurons of the CNS suggesting a possible link to the FOXG1 syndrome pathology [2086]
KCNE1 and KCNE3
KCNE1 and KCNE3 beta-subunits regulate membrane surface expression of kv12.2 channels in vitro and form tripartite complex in vivo [801]
E4031
RELK1 and RELK2 currents were not blocked by 10 μm E4031 (n = 5), which blocks HERG channels, nor by 10 μm linopirdine (n = 5), which blocks M-channels [809]
References
Saganich MJ
et al.
Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain.
J. Neurosci.,
2001
Jul
1
, 21 (4609-24).
Gutman GA
et al.
International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels.
Pharmacol. Rev.,
2005
Dec
, 57 (473-508).
Meves H
et al.
Separation of M-like current and ERG current in NG108-15 cells.
Br. J. Pharmacol.,
1999
Jul
, 127 (1213-23).
Clancy SM
et al.
KCNE1 and KCNE3 beta-subunits regulate membrane surface expression of Kv12.2 K(+) channels in vitro and form a tripartite complex in vivo.
PLoS ONE,
2009
, 4 (e6330).
Noma K
et al.
Triple N-glycosylation in the long S5-P loop regulates the activation and trafficking of the Kv12.2 potassium channel.
J. Biol. Chem.,
2009
Nov
27
, 284 (33139-50).
Zhang X
et al.
Deletion of the potassium channel Kv12.2 causes hippocampal hyperexcitability and epilepsy.
Nat. Neurosci.,
2010
Sep
, 13 (1056-8).
Zou A
et al.
Distribution and functional properties of human KCNH8 (Elk1) potassium channels.
Am. J. Physiol., Cell Physiol.,
2003
Dec
, 285 (C1356-66).
Engeland B
et al.
Cloning and functional expression of rat ether-à-go-go-like K+ channel genes.
J. Physiol. (Lond.),
1998
Dec
15
, 513 ( Pt 3) (647-54).
Miyake A
et al.
New ether-à-go-go K(+) channel family members localized in human telencephalon.
J. Biol. Chem.,
1999
Aug
27
, 274 (25018-25).
Smith GA
et al.
Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells.
J. Biol. Chem.,
2002
May
24
, 277 (18528-34).
Ganetzky B
et al.
The eag family of K+ channels in Drosophila and mammals.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (356-69).
Becchetti A
et al.
The functional properties of the human ether-à-go-go-like (HELK2) K+ channel.
Eur. J. Neurosci.,
2002
Aug
, 16 (415-28).
Grosso S
et al.
Epilepsy and electroencephalographic findings in pericentric inversion of chromosome 12.
J. Child Neurol.,
2004
Aug
, 19 (604-8).
Miyake A
et al.
Disruption of the ether-a-go-go K+ channel gene BEC1/KCNH3 enhances cognitive function.
J. Neurosci.,
2009
Nov
18
, 29 (14637-45).
Kazmierczak M
et al.
External pH modulates EAG superfamily K+ channels through EAG-specific acidic residues in the voltage sensor.
J. Gen. Physiol.,
2013
Jun
, 141 (721-35).
Vezzali R
et al.
The FOXG1/FOXO/SMAD network balances proliferation and differentiation of cortical progenitors and activates Kcnh3 expression in mature neurons.
Oncotarget,
2016
May
21
, ().
Contributors: Rajnish Ranjan, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/39/ , accessed on 2026 Mar 01