Kir7.1
Description: potassium inwardly-rectifying channel, subfamily J, member 13 Gene: Kcnj13 Alias: Kir7.1, kcnj13
A large variety of potassium channels have been recognized to be of physiological importance in the kidney. Several of those belong to the family of inward rectifier K+ channels (Kir channels) that has been classified into seven subgroups (Kir1–7) (Reimann [1069]). A renal member of this family is the Kir7.1 channel.
The gene KCNJ13 (also known as SVD; KIR1.4; KIR7.1; MGC33328) encodes Kir7.1, a member of the inwardly rectifying potassium channel family, subfamily J, member 13. Members of this family form ion channel pores that allow potassium ions to pass into a cell. The encoded protein belongs to a subfamily of low signal channel conductance proteins that have a low dependence on potassium concentration. Mutations in this gene are associated with snowflake vitreoretinal degeneration. Alternate splicing results in multiple transcript variants.
http://www.ncbi.nlm.nih.gov/gene/3769
Experimental data
Rat Kir7.1 gene in CHO host cells |
||
|
Click for details 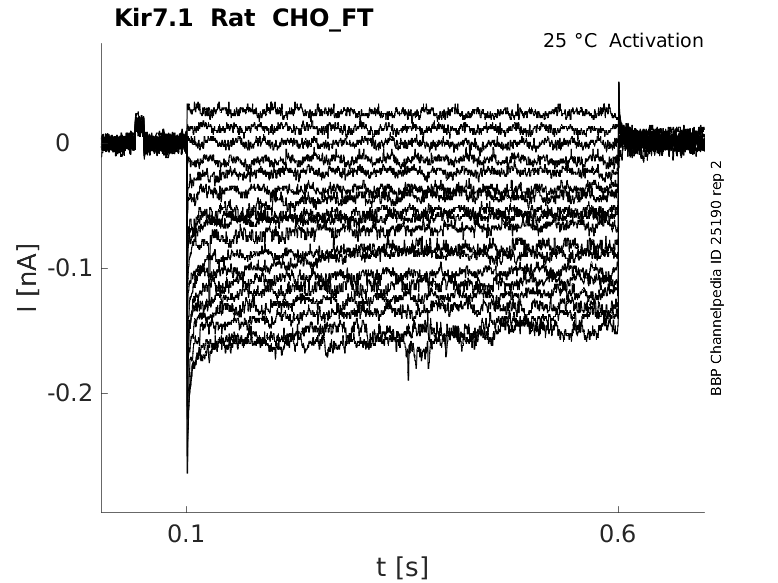
25 °Cshow 35 cells |
Click for details 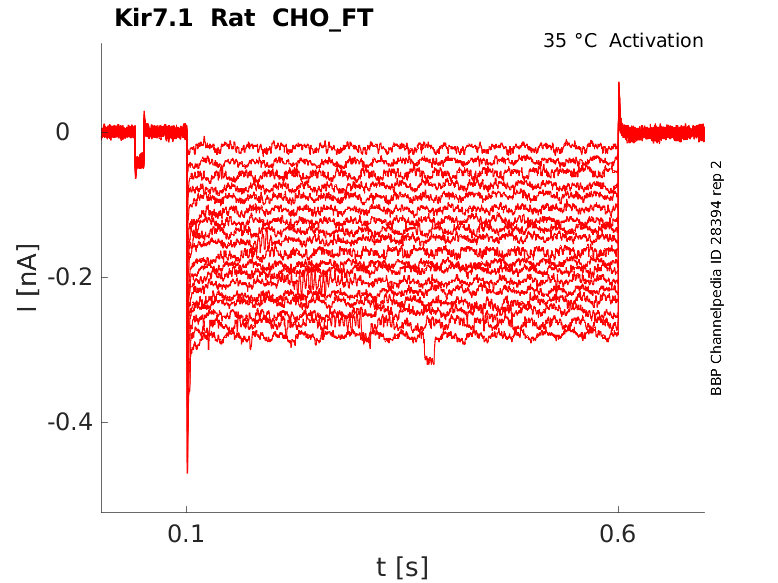
35 °Cshow 30 cells |
|
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_002242.4 | 3609 | |
| Mouse | NM_001110227.2 | 3056 | |
| Rat | NM_053608.2 | 1268 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Structure
Kir7.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Kir7.1 displays unique biophysical characteristics, particularly a very weak inward rectification and a lack of major influence of external K+ on channel conductance, both being markedly different from other members of Kir channels (Derst [1070]).
Kir7.1 is located predominantly in the basolateral membrane of the proximal tubule (PT), the thick ascending limb (TAL) (Derst [1070]) and the cortical collecting duct (CCD) principal cells (Ookata[1071]).
The expression of Kir7.1 in the brain appears to be restricted to the epithelial cells of the choroid plexus (Doring [1042], Nakamura [1073]). It is also strongly expressed in various other epithelial cells, for example, in small intestine or in follicular cells of the thyroid gland. Significant expression of gpKir7.1 was found in brain, kidney, and lung, but not in heart, skeletal muscle, liver, or spleen. (Derst [1070])
Immunocytochemical detection in guinea pig identified the gpKir7.1 protein in the basolateral membrane of epithelial cells of the proximal tubule. RT-PCR analysis identified strong gpKir7.1 expression in the proximal tubule and weak expression in glomeruli and thick ascending limb. In isolated human tubule fragments, RT-PCR showed expression in proximal tubule and thick ascending limb. (Derst [1070])
Kir7.1 it has been proposed to contribute to tubular K+ recycling and secretion (Derst [1070], Ookata [1071], Doring [1072]). The evidence that expression of Kir7.1 is upregulated under dietary potassium overload also supports a functional significance for K+ secretion (Ookata [1071]).
the dual regulation of Kir7.1 channel function by protein kinase A (PKA) and protein kinase C (PKC). Structurally, these regulations depend on two key residues in the C-terminal channel domain (Ser201 for PKC and Ser287 for PKA). (Zahng [1067])
Low micromolar concentrations of the small molecule VU590 inhibit Kir7.1, as an intracellular pore blocker. (Lewis [1068])
The expression of gpKir7.1 in Xenopus laevis oocytes revealed inwardly rectifying K+ currents. The reversal potential was strongly dependent on the extracellular K+ concentration, shifting from -14 mV at 96 mmol/L K+ to -90 mV at 1 mmol/L K+. gpKir7.1 showed a low affinity for Ba2+. (Derst [1070])
References
Zhang W
et al.
Dual regulation of renal Kir7.1 potassium channels by protein Kinase A and protein Kinase C.
Biochem. Biophys. Res. Commun.,
2008
Dec
19
, 377 (981-6).
Lewis LM
et al.
High-throughput screening reveals a small-molecule inhibitor of the renal outer medullary potassium channel and Kir7.1.
Mol. Pharmacol.,
2009
Nov
, 76 (1094-103).
Reimann F
et al.
Inwardly rectifying potassium channels.
Curr. Opin. Cell Biol.,
1999
Aug
, 11 (503-8).
Derst C
et al.
Cellular localization of the potassium channel Kir7.1 in guinea pig and human kidney.
Kidney Int.,
2001
Jun
, 59 (2197-205).
Ookata K
et al.
Localization of inward rectifier potassium channel Kir7.1 in the basolateral membrane of distal nephron and collecting duct.
J. Am. Soc. Nephrol.,
2000
Nov
, 11 (1987-94).
Döring F
et al.
The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties.
J. Neurosci.,
1998
Nov
1
, 18 (8625-36).
Nakamura N
et al.
Inwardly rectifying K+ channel Kir7.1 is highly expressed in thyroid follicular cells, intestinal epithelial cells and choroid plexus epithelial cells: implication for a functional coupling with Na+,K+-ATPase.
Biochem. J.,
1999
Sep
1
, 342 ( Pt 2) (329-36).
Credits
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/55/ , accessed on 2026 Feb 28