Kv9.3
Description: potassium voltage-gated channel, delayed-rectifier, subfamily S, member 3 Gene: Kcns3 Alias: Kv9.3, kcns3
Kv9.3 (also known as MGC9481), encoded by the gene HCNS3, is member 3 of subfamily S of delayed-rectifier potassium voltage-gated channels. Kv9.3 is not functional by itself but can form heteromultimers with member 1 and with member 2 (and possibly other members) of the Shab-related subfamily of potassium voltage-gated channel proteins. NCBI
Experimental data
Rat Kv9.3 gene in CHO host cells datasheet |
||
|
Click for details 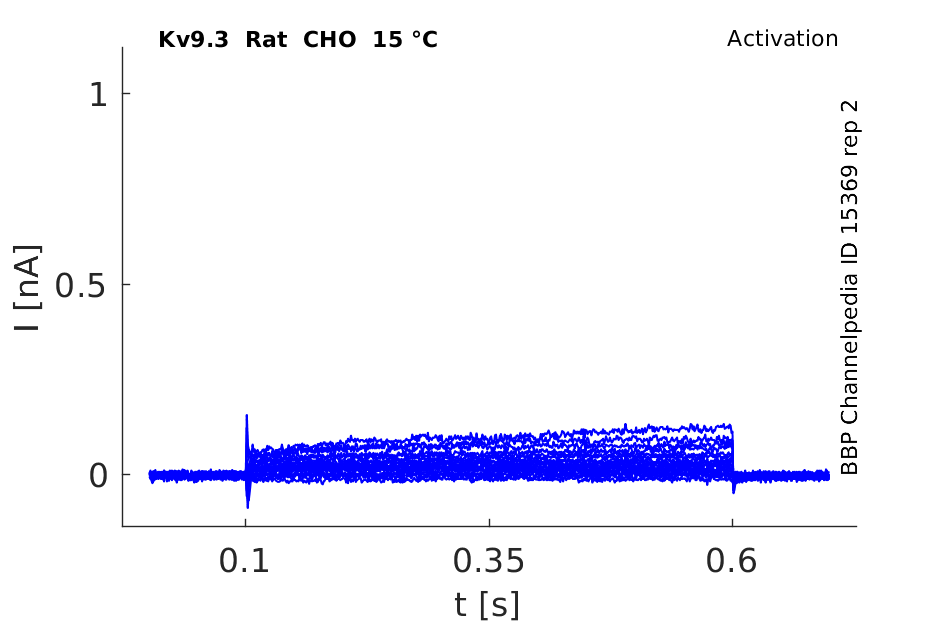
15 °Cshow 59 cells |
Click for details 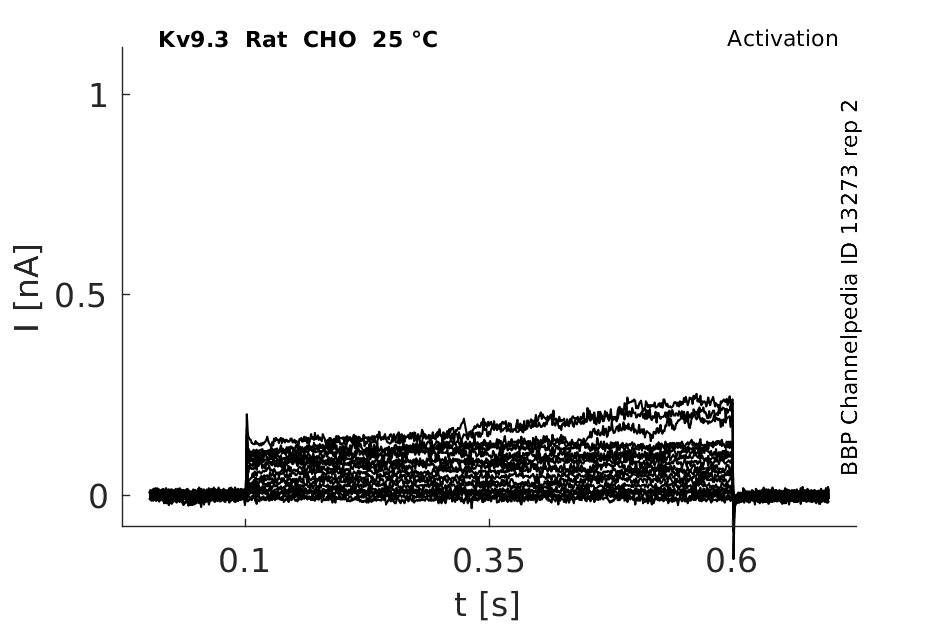
25 °Cshow 76 cells |
Click for details 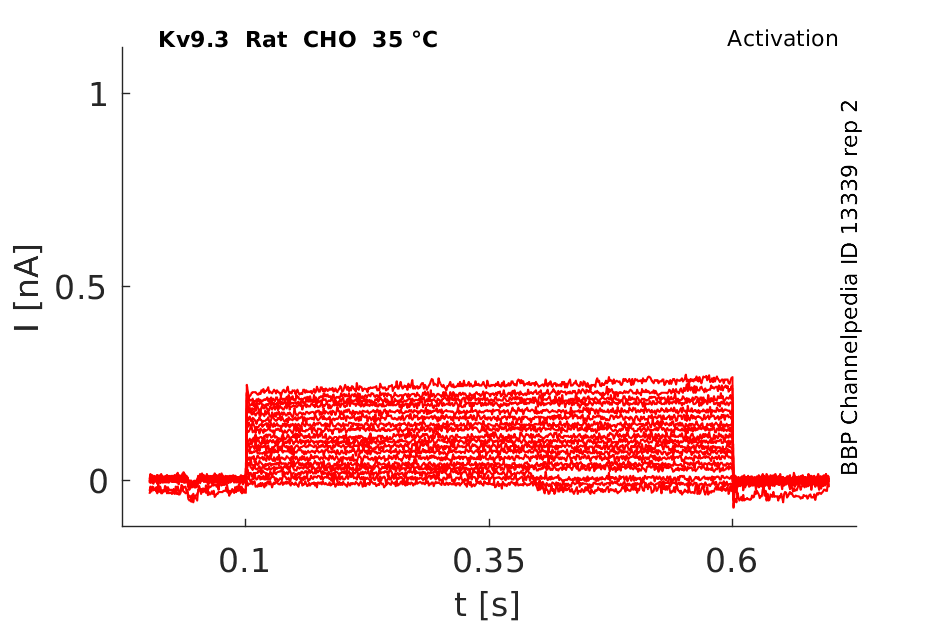
35 °Cshow 94 cells |
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_002252.5 | 2341 | |
| Mouse | NM_173417.3 | 2933 | |
| Rat | NM_031778.3 | 2311 |
Similarity to Kv9.1
The identity between Kv9.1 and Kv9.3 is only 54% within this conserved region, a rather low value in comparison with percentages higher than 70% found comparing members within the Kv1, Kv2 or Kv3 sub- families. If one would use 70% as a criterium to define subfamily membership, Kv9.3 could actually be a member of a new family, Kv10.1. The future cloning of additional homologue a-subunits will decide if Kv9.1 and Kv9.3 belong to the same or to two subfamilies [401]
Isoforms
Post-Translational Modifications
Visual Representation of Kv9.3 Structure
Methodology for visual representation of structure available here
Kv9.3 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Kv9.3 heteromer with Kv2.1 in X.oocytes


Kv9.3 Co-transfected with Kv2.1 in CHO cells

Interaction of Kv9.3 and Kv2.1 in the Heart
A degenerate PCR-based strategy identified Kv1.2, Kv1.3, Kv2.1, and a novel K+ channel, Kv9.3, as potential oxygen-sensitive PA K+ channels. FIGURE 1F shows the expression of the various channels in lung, brain, conduit, and resistance pulmonary arter- ies by RT-PCR. Kv9.3 transfected cells did not express any exogenous channel activity. When Kv9.3 was coexpressed with Kv2.1, the activation threshold was shifted toward negative values (–50 mV), and the amplitude of the currents was greatly enhanced. Examination of the single-channel properties of Kv2.1 and Kv2.1/Kv9.3 revealed that Kv9.3 alters the single channel conductance of Kv2.1. The activity of both Kv2.1 and Kv2.1/Kv9.3 in excised inside-out patches was sensitive to the presence of internal ATP. When ATP concentration was lowered from 5 mM to 1 mM, channel activity was reduced by 55% [1851]
Interaction of Kv9.3 with Kv2.1 (rat) in X.oocytes
Kv9.3 increased the single-channel conductance of Kv2.1, from 8.5 to 14.5 pS. At the pharmacological level, the sensitivity to both 4-AP and TEA were reduced when Kv2.1 was co-expressed with Kv9.3. The alterations in the biophysical and pharmacological properties of Kv2.1 by Kv9.3 suggest that these two subunits associate to form a heteromultimer whose properties are different from that of the Kv2.1 [1871]
Kv9.3 Expression in Lungs
Kv9.1 is exclusively expressed in the brain, whereas Kv9.3 is more widely expressed, with the highest expression level in the lung [401]
Kv9.3 Function in Brain
Stromatoxin-sensitive, heteromultimeric Kv2.1/Kv9.3 channels contribute to myogenic control of cerebral arterial diameter [1849]
Kv9.3 Function in Rat Brain
The Kv2.1/Kv9.3 heteromer generates an O2 sensitive potassium channel and induces a slow deactivation that has important consequences for brain and lung physiology. We examined the developmental regulation of Kv2.1 and Kv9.3 mRNAs in brain and lung. Both genes followed parallel expression patterns in brain, increasing progressively through post-natal life. In lung, however, the expression of the two genes followed opposite trends: Kv2.1 transcripts decreased, while Kv9.3 mRNA increased. The Kv9.3/Kv2.1 ratio shows that while in brain the expression of both genes followed a similar pattern, the relative abundance of Kv9.3 increased steadily through post-natal life in lung. Furthermore, there is selective regulation of gene expression during the suckling-weaning transition. Our results suggest that different Kv9.3/Kv2.1 ratios could have physiological implications in both organs during post-natal development, and that diet composition and selective tissue-specific insulin regulation modulate the expression of Kv2.1 and Kv9.3 [1850]
Potassium channels form the most diverse class in the ion channel superfamily, giving rise to a large variety of currents, the kinetics of which are shaped to the requirements of their physiological function (Hille, B. 1992. Ionic Channels of Excitable Membranes. Sinauer Associates, Sunderland, MA). Part of this diversity is of combinatorial origin, inasmuch as potassium channels are oligomeric protein complexes [580], [744]. Participation of different proteins occurs by two mechanisms. Either distinct alpha-subunits assemble into heterotetrameric channels with all subunits lining the pore [741], [649], [745] or auxiliary beta-subunits associate with tetrameric channel complexes, changing their kinetic properties [312]. Modulatory alpha-subunits form a group of proteins that contributes to the diversity of potassium channels by the first mechanism. [177]
Kv9.2 is an alpha-subunit, highly similar to other Kv alpha-subunits by primary sequence, yet unable to form homomeric conducting channels in heterologous expression systems [746], [726], [400], [401].
Specific N-terminal interactions between Kv2- and modulatory alpha-subunits promote the assembly of heterotetrameric channels [399], [398], [747] displaying altered characteristics in comparison to homomeric Kv2 channels [726], [4, [748], [398].
References
Kerschensteiner D
et al.
Heteromeric assembly of Kv2.1 with Kv9.3: effect on the state dependence of inactivation.
Biophys. J.,
1999
Jul
, 77 (248-57).
Rettig J
et al.
Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit.
Nature,
1994
May
26
, 369 (289-94).
Kramer JW
et al.
Modulation of potassium channel gating by coexpression of Kv2.1 with regulatory Kv5.1 or Kv6.1 alpha-subunits.
Am. J. Physiol.,
1998
Jun
, 274 (C1501-10).
Post MA
et al.
Kv2.1 and electrically silent Kv6.1 potassium channel subunits combine and express a novel current.
FEBS Lett.,
1996
Dec
9
, 399 (177-82).
Salinas M
et al.
New modulatory alpha subunits for mammalian Shab K+ channels.
J. Biol. Chem.,
1997
Sep
26
, 272 (24371-9).
Stocker M
et al.
Cloning and tissue distribution of two new potassium channel alpha-subunits from rat brain.
Biochem. Biophys. Res. Commun.,
1998
Jul
30
, 248 (927-34).
MacKinnon R
Determination of the subunit stoichiometry of a voltage-activated potassium channel.
Nature,
1991
Mar
21
, 350 (232-5).
Isacoff EY
et al.
Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes.
Nature,
1990
Jun
7
, 345 (530-4).
Hugnot JP
et al.
Kv8.1, a new neuronal potassium channel subunit with specific inhibitory properties towards Shab and Shaw channels.
EMBO J.,
1996
Jul
1
, 15 (3322-31).
Christie MJ
et al.
Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits.
Neuron,
1990
Mar
, 4 (405-11).
Parcej DN
et al.
Oligomeric properties of alpha-dendrotoxin-sensitive potassium ion channels purified from bovine brain.
Biochemistry,
1992
Nov
17
, 31 (11084-8).
Ruppersberg JP
et al.
Heteromultimeric channels formed by rat brain potassium-channel proteins.
Nature,
1990
Jun
7
, 345 (535-7).
Drewe JA
et al.
Distinct spatial and temporal expression patterns of K+ channel mRNAs from different subfamilies.
J. Neurosci.,
1992
Feb
, 12 (538-48).
Stocker M
et al.
Subunit assembly and domain analysis of electrically silent K+ channel alpha-subunits of the rat Kv9 subfamily.
J. Neurochem.,
1999
Apr
, 72 (1725-34).
Castellano A
et al.
Identification and functional characterization of a K+ channel alpha-subunit with regulatory properties specific to brain.
J. Neurosci.,
1997
Jun
15
, 17 (4652-61).
Shepard AR
et al.
Electrically silent potassium channel subunits from human lens epithelium.
Am. J. Physiol.,
1999
Sep
, 277 (C412-24).
Zhong XZ
et al.
Stromatoxin-sensitive, heteromultimeric Kv2.1/Kv9.3 channels contribute to myogenic control of cerebral arterial diameter.
J. Physiol. (Lond.),
2010
Nov
15
, 588 (4519-37).
Coma M
et al.
Different Kv2.1/Kv9.3 heteromer expression during brain and lung post-natal development in the rat.
J. Physiol. Biochem.,
2002
Dec
, 58 (195-203).
Patel AJ
et al.
Kv2.1/Kv9.3, an ATP-dependent delayed-rectifier K+ channel in pulmonary artery myocytes.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (438-41).
Patel AJ
et al.
Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes.
EMBO J.,
1997
Nov
17
, 16 (6615-25).
Contributors: Rajnish Ranjan, Michael Schartner, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/32/ , accessed on 2026 Feb 27