Kv7
The KCNQ gene family encodes Kv7 channel subunits that form homo- or heteromeric Kv7 channels (also termed M-channels) which represent the molecular correlate to the M-current [695].
KCNQ STRUCTURE
KCNQ proteins have six transmembrane domains and are structurally related to Kv potassium channels. The degree of homology between different KCNQ proteins is less than that observed within Kv family branches (for example, within Kv1 channels). Like other Kv channels, KCNQ subunits have a single P-loop that forms the selectivity filter of the pore (in four copies provided by four subunits), a positively charged fourth transmembrane domain (S4) that probably acts as a voltage sensor, and intracellular amino and carboxy termini. The C terminus is quite long, and contains a conserved domain (the 'A domain'24) closely followed by a short stretch thought to be involved in subunit assembly, at least in KCNQ1 [463]
KCNQ General Kinetics
Kv7 subtypes start opening at around −60 mV (although the voltage dependency of Kv7 channels differ between heterologous and native cells), thus being functionally active close to the resting membrane potential. In addition, Kv7 channels may be considered as being non-inactivating (‘leaky’) K+ channels at physiologically relevant resting potentials, although a proportion of Kv7 channels may undergo steady-state inactivation [712]. These characteristics enable the Kv7 channels to produce the underlying subthreshold M-current, which stabilizes the neuronal resting potential. Consequently, the Kv7 channels are thought to inhibit neuronal excitability and put a ‘brake’ on action potential firing when the neuron is exposed to an excitatory stimulus. [752]
M Current
M currents are a subset of native K+ currents recorded from various neurones such as sympathic, hippocampal and cortical neurones (Marrion, 1997 [461]). These currents are voltage-dependent currents activated by depolarisation, they are non-inactivating, blocked by muscarinic M1 receptor stimulation and serve to stabilize the membrane potential, and thus, reduce neuronal excitability. KCNQ channels have been suggested to be the molecular counterpart of the M channel and M-like channels (Wang et al., 1998 [695]; Schroeder et al., 2000 [136]).
Brown and colleagues were the first to describe a neuronal K+ current in bullfrog sympathetic cells that they named ‘M current’ due to its suppression by stimulation of muscarinic acetylcholine receptors (mAChRs) [465]. This time- and voltage-dependent K+ current has a threshold for activation at typical neuronal resting potentials, with greater activity upon depolarization. This characteristic and its lack of inactivation give M current a major impact on neuronal excitability. Suppression of M current by the activation of appropriate receptors or by pharmacological blockade allows neurons to fire more rapidly due to the reduction in accommodation or spike frequency adaptation [758], [695], [759].
Biophysics
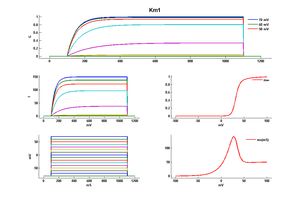
Model Km1 (ID=15)
| Animal | N.A | |
| CellType | N.A | |
| Age | 0 Days | |
| Temperature | 0.0°C | |
| Reversal | -84.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [268] A Bibbig et. al; J. Neurosci. 2001 Nov 15 | |
| mpower | 1.0 | |
| m Alpha | 0.02/(1+exp((40-v)/5)) | |
| m Beta | 0.01*exp((17-v)/18) | |
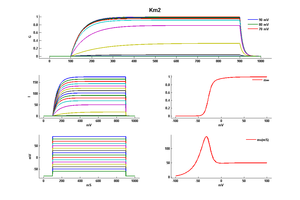
Model Km2 (ID=16)
Original Kfast model
| Animal | N.A | |
| CellType | N.A | |
| Age | 0 Days | |
| Temperature | 0.0°C | |
| Reversal | -84.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [269] Roger D Traub et. al; J. Neurophysiol. 2003 Feb | |
| mpower | 1.0 | |
| m Alpha | 0.02/(1+exp((-v-20)/5)) | |
| m Beta | 0.01*exp((-v-43)/18) | |
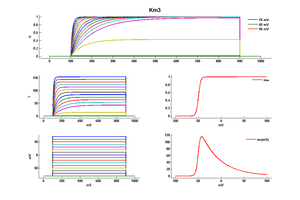
Model Km3 (ID=17)
| Animal | N.A | |
| CellType | N.A | |
| Age | 0 Days | |
| Temperature | 0.0°C | |
| Reversal | -84.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [270] D Johnston et. al; J. Neurophysiol. 1995 Mar | |
| mpower | 1.0 | |
| m Alpha | 0.006*exp(10*0.06*(v+55)*0.04) | |
| m Beta | 0.06*exp(-9.4*(v+55)*0.04) | |
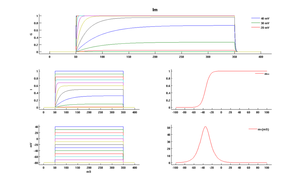
Model Im (ID=47)
| Animal | Bullfrog | |
| CellType | Neurons | |
| Age | 0 Days | |
| Temperature | 0.0°C | |
| Reversal | -85.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [1498] D A Brown et. al; J. Physiol. (Lond.) 1982 Sep | |
| mpower | 1.0 | |
| m Alpha | 3.3e-3 * exp(2.5 * 0.04*(v - -35)) | |
| m Beta | 3.3e-3 * exp(-2.5 * 0.04 *(v - -35)) | |
Kv7 channels are inhibited by stimulation of a variety of Gq/11 -coupled neurotransmitter receptors. [751]
References
Schrøder RL
et al.
KCNQ4 channel activation by BMS-204352 and retigabine.
Neuropharmacology,
2001
Jun
, 40 (888-98).
Brown DA
et al.
Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone.
Nature,
1980
Feb
14
, 283 (673-6).
Wang HS
et al.
KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel.
Science,
1998
Dec
4
, 282 (1890-3).
Pan Z
et al.
A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon.
J. Neurosci.,
2006
Mar
8
, 26 (2599-613).
Jensen HS
et al.
Inactivation as a new regulatory mechanism for neuronal Kv7 channels.
Biophys. J.,
2007
Apr
15
, 92 (2747-56).
Hernandez CC
et al.
Regulation of neural KCNQ channels: signalling pathways, structural motifs and functional implications.
J. Physiol. (Lond.),
2008
Apr
1
, 586 (1811-21).
Hansen HH
et al.
Kv7 channels: interaction with dopaminergic and serotonergic neurotransmission in the CNS.
J. Physiol. (Lond.),
2008
Apr
1
, 586 (1823-32).
Jones S
et al.
Bradykinin excites rat sympathetic neurons by inhibition of M current through a mechanism involving B2 receptors and G alpha q/11.
Neuron,
1995
Feb
, 14 (399-405).
Zaika O
et al.
Inositol triphosphate-mediated Ca2+ signals direct purinergic P2Y receptor regulation of neuronal ion channels.
J. Neurosci.,
2007
Aug
15
, 27 (8914-26).
Gutman GA
et al.
International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels.
Pharmacol. Rev.,
2003
Dec
, 55 (583-6).
Adams PR
et al.
M-currents and other potassium currents in bullfrog sympathetic neurones.
J. Physiol. (Lond.),
1982
Sep
, 330 (537-72).
Credits
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/213/ , accessed on 2026 Feb 16