HCN
Alias: Ih
The hyperpolarization-activated cyclic nucleotide–gated (HCN) channels belong to the family of the voltage-gated pore loop channels and are composed of four members. They play a major role in generating neuronal and cardiac automaticity, where their current is generally referred to as I(f) in the heart and I(h )in the brain [2292] [457]
In mammals, the four functionally expressed hyperpolarization-activated cyclic nucleotide-gated channels (HCN1-HCN4) are encoded by 4 genes [2293]. These are, respectively:
HCN1 : hcn1
HCN2 : hcn2
HCN3 : hcn3
HCN4 : hcn4
The four channels encoding genes are related through roughly 80-90% sequence identity [2294] [2293].
All HCN channels have a canonical mRNA transcript coding for the canonical ion channel protein and no HCN transcript variants have been identified.
When referring to a particular HCN, one generally means the canonical isoforms. To date, no HCN isoforms have been identified.
When comparing between species, HCN sequences show high amino sequence homology. The rat HCN1-4 were demonstrated to have > 97% identity to mouse HCN1-4 channels. [337]
Like all mammalian proteins, HCN channels are subject to post translational modification.
These include, but are not limited to:
- Phosphorylation [2295] [2297] [457]
- Glycosylation [2296] [457]
- Ubiquitination [2293]
- S-palmitoylation [2298]
- SUMOylation [2293]
The functional effects of channel phosphorylation on HCN electrophysiology often depend on the specific HCN subtype. Research on PTMs has highlighted how they can alter trafficking, localization, gating, and pharmacology, among other properties.
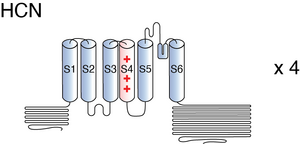
Visual Representation of HCN General Structure
Methodology for visual representation of structure available here
HCN channels are comprised of four subunits, arranged around a centrally located pore. Each subunit is divided into three structural elements: the transmembrane core, the cytosolic N-terminal, and the COOH-terminal. [457]
The transmembrane segment of every subunit is made of six transmembrane helices (S1–S6), with a positively charged voltage sensor (S4). S4 carries nine arginine or lysine residues regularly spaced at every third position. Inward movement of S4 leads to opening of HCN channels [457]. S5 and S6 form the pore region where the ion selectivity filter is present [2295]. HCN’s selectivity for K+ is conferred by a glycine-tyrosine-glycine (GYG) motif located in this pore region. Despite an expected selectivity for only K+, HCN Na+ permeability can be structurally explained as the filter adopts a non-canonical conformation that contains two instead of four cation binding sites, allowing for the passage of Na+. [457] [2299]
Past S6 is the COOH-terminal that harbors the C-linker, the cyclic nucleotide binding domain (CNBD), and C-terminal. The C-linker is a 80-residue segment consisting of six α-helices (A′–F′). The CNBD consists of three α-helices (A–C) and a β-roll between the A- and B-helices Together, the C-linker and CBND are sometimes referred to as the “cAMP-sensing domain” (CSD) [2295] The transmembrane regions and the proximal COOH terminus allosterically interact with each other during channel gating and reveal a high degree of sequence conservation within the HCN channel family. In contrast, cytosolic N-terminal and the sequence downstream of the CNBD vary considerably in their length and share only modest to low homology between various HCN channels [457]
Several unique structural features could explain the reversed polarity of HCN channels voltage-dependent gating. These include:
- An unusually elongated S4 helix that interfaces with a C-linker within the intracellular environment. This interaction serves to stabilize the closed state of the channel when the voltage sensor is in a depolarized state (positively charged).
- The spatial arrangement of the S4, S5, and S6 helices which facilitates the voltage sensor's ability to uphold a closed pore configuration when the membrane voltage is depolarized.
- The presence of an HCN domain that further reinforces the closed state of the pore when the voltage sensor is depolarized.
These structural features could explain the mechanisms by which hyperpolarization induced inward movement of S4 would disrupt these stabilizing interactions, allowing the S6 helices to spontaneously open [2299]
The members of the HCN family generally exhibit similarities in their kinetics, although there are distinctions in the speed of activation and deactivation between individual HCN subtypes. HCN1 is characterized as the fastest among HCN channels, followed by HCN2, HCN3, and finally, HCN4. These variations in kinetics speed can be attributed to structural differences within each individual protein, underscoring the impact of protein structure on channel function and behavior. [2300]
The current passing through HCN channels is often referred to as I(h), standing for hyperpolarization-activated current, or I(f), denoting funny current. The former term is commonly used in the context of the nervous system, while the latter is more frequently employed in the context of the cardiac system.
A noteworthy characteristic of HCN channel kinetics is their reversed polarity. Unlike most voltage-gated ion channels, HCN channels are activated by membrane hyperpolarization, typically at negative potentials in the range of approximately -50 to -60 mV, and they do not exhibit voltage-dependent inactivation. Typically, the activation of I(h) (or I(f)) involves two kinetic components: a minor instantaneous current (IINS) that achieves full activation within a few milliseconds, and a major, slowly developing component (ISS) that reaches its steady-state level over a range of tens of milliseconds to several seconds under fully activating conditions. [2292] [457]
HCN channels display other interesting kinetic properties. First, HCN channels are constitutively open at voltages near the resting membrane potential and this contributes to the setting of the resting membrane potential. Second, because reversed polarity behavior, HCN channels counteract both membrane hyperpolarization and depolarisation by producing either a depolarising inward current, upon activation, or by facilitating hyperpolarisation, upon deactivation. These properties lead to the dampening of inhibitory and excitatory stimulus inputs to the cell and stabilize membrane potential [457]
Voltage hysteresis refers to the phenomenon where the activation or behavior of a channel varies when the voltage is increasing (i.e., transitioning from lower to higher voltage) compared to when it is decreasing (i.e., transitioning from higher to lower voltage). This discrepancy in channel response during voltage changes can have important implications for its functional properties. These channels may open or close at different voltage levels when the voltage is ramped up as opposed to when it's ramped down. HCN channels generally have 2 modes: Mode I and Mode II. In Mode I, the movement of electric charges and the opening of the channel happen at very negative (lower) voltages. In Mode II, these processes occur at more positive (higher) voltages, typically over 50 millivolts higher. The channel switches between these two modes, favoring Mode I when it is already open and preferring Mode II when it's closed. This switching between modes affects how quickly the channel turns on and off. [457]
Single channel unitary conductance
Single channel unitary conductance refers to the conductance of an individual ion channel when it is open and is typically measured in siemens (S) or pico siemens (pS). The conductance value is influenced by various factors, including the size and charge of the ions, as well as the properties of the channel itself, such as its structure, selectivity, and gating mechanisms.
The single channel unitary conductance of HCN is still a subject of debate. Originally, single-channel conductance was found to be very low, in the range of ∼1 pS. However, other experiments have recorded single-channel conductances that are 10–30 times higher. It is unclear how this major discrepancy can be explained. It is possible that single-channel unitary conductance may be dynamically regulated by modulatory factors and proteins assembled with HCN channels in vivo. [457]
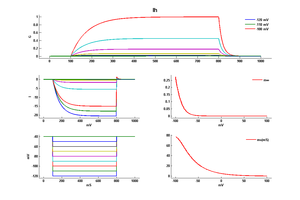
Model
Model Ih (ID=14)
| Animal | rat | |
| CellType | Neocortical L5PC | |
| Age | 50 Days | |
| Temperature | 34.0°C | |
| Reversal | -45.0 mV | |
| Ion | Hcn + | |
| Ligand ion | ||
| Reference | [263] Maarten H P Kole et. al; J. Neurosci. 2006 Feb 8 | |
| mpower | 1.0 | |
| m Alpha | 0.001*6.43*(v+154.9)/(exp((v+154.9)/11.9)-1) If v neq -154.9 | |
| m Beta | 0.001*193*exp(v/33.1) | |
HCN channels are located throughout the body but are predominantly located in the nervous system [2293]:
- HCN1 can be found in the neocortex, hippocampus, cerebellar cortex, brainstem, and spinal cord (CNS) and the retina and the dorsal root ganglion (PNS).
- HCN2 is ubiquitous across the CNS, but especially abundant in thalamic and brainstem nuclei. In the PNS, it can be found in the DRG and sensory neurons.
- HCN3 is scattered at low levels throughout the brain.
- HCN4 is predominantly located in the Sinoatrial node of cardiac pacemaker cells.
Though mainly found in the aforementioned areas, the expression of the specific HCNs is not restricted to those sole locations. Indeed, the distribution of a particular HCN will depend on its subtype. Please refer to the specific page for more detailed information.
The subcellular distribution of HCNs will depend on the particular HCN subtype. Please refer to the specific page for more detailed information.
However, HCN channels are generally predominantly found in the dendrites of neural cells [2301].
Given their kinetic properties and widespread locations, HCN channels are responsible for a number of functions related to pacemaking in cells and tissues. These include [457] [2295] :
- Controlling resting membrane potential and neuronal oscillation in the soma and proximal dendrites
- Dendritic integration
- Integration in the presynaptic terminals
- Working memory
- Resonance and Oscillation
- Generation of thalamic rhythms
- Maintenance of pacemaking in cardiac cells [2292]
Though often involved in those roles, individual HCN channels may perform additional functions depending on the channel subtype. Please refer to the individual pages for more details on the specific role of an individual HCN channel.
Channelopathies
Mutations in any of the genes encoding HCN channels can alter their biophysical properties leading to the development of channelopathies. Channelopathies result from autosomal dominant inheritance and de novo mutations. Such aberrations are often the cause for the following pathologies [2295] [2292]:
- Epilepsy & related seizure disorders
- Cardiac remodeling and arrhythmia
- Neuropathic pain
HCN channels are regulated by various auxiliary proteins and secondary messengers. These regulatory proteins play important roles in the development, localization, and expression of the channel protein.
Heteromeric HCNs
Though protein isoforms [clickable link to Isoform section] resulting from alternative splicing have not been confirmed, there is evidence that different HCN isoform subunits can assemble to form function heteromeric channels. These heteromeric proteins have intermediate properties compared to their homomeric counterparts and could serve as a “fine-tuning” of HCN current in those tissues. The expression of these heteromeric channels generally occurs in areas where the expression of the different channel canonical isoforms / members overlap. A prominent example is HCN1 and HCN2, where their locations in the CNS overlap and hybrid channels have been identified in vivo. (31914897)
Cyclic nucleotides
Cyclic adenosine monophosphate (cAMP) is a major modulator of HCN channels. It primarily affects HCN2 and HCN4 subtypes, right shifting their voltage-dependent activation curves by about +17 mV, increasing opening kinetics. The effect of cAMP on HCN1 and HCN3 is weaker, shifting the activation curve by only 2-4 mV.
Other cyclic nucleotides also affect HCN channels, though to a lesser degree than cAMP. cGMP and cCMP both modulate HCN, particularly HCN2 and HCN4. They induce a positive shift of about 6-8 mV. [457] [2302] [2295]
Regulation of HCN channels by cAMP is mediated by the proximal portion of the cytosolic COOH terminus, namely the CNBD and C-linker regions. When all 4 HCN subunits assemble in the absence of cyclic nucleotides, the 4 CNBD/C-linkers form a non-tetrameric complex that is thought to exert a tonic inhibition on the pore of the channel [2303]. When the cAMP is present, the C-linker/CNBD region tetramerizes and releases the inhibition of the pore gate, adopting a “looser conformation” that allows easier passage of ions. In channels such as HCN1, the C-terminal domain is already in a tetramerised state, even at basal cAMP concentrations, in contrast to HCN2 and HCN4, which require saturating cAMP levels for tetramerization. The differences in inherent tetramerization between the different channels is what determines the modulation impact of cAMP [2304] [2300] [457]
Sinus node inhibitors
Sinus node inhibitors are compounds that block HCN channels, thus eliminating Ih/If current. These blockers include cilobradine, ivabradine, and zatebradine and are often used clinically to reduce heart rate. [2305]
Other molecules
Aside from these major interacting compounds, there are a whole host of other compounds that have been shown to interact and influence the properties of HCN channels. These include but are not limited to:
Small Molecules [2295]
Membrane acidic lipids:
- Phosphatidylinositol-4, 5-bisphosphate (PIP2): PIP2 shifts the voltage-dependent activation of HCN channels by about -20 mV.
- Phosphatidic Acid (PA) and Arachidonic Acid (AA): shift the voltage-dependent activation of HCN channels by approximately + 5-10 mV.
Intracellular protons:
Intracellular acidification shifts the voltage-dependent activation of HCN channels negatively and reduces activation rate (downregulation of Ih). Conversely, alkalinization shifts voltage-dependent activation of HCN channels positively and increases activation rate (enhancement of Ih).
Acetazolamide: a carbonic anhydrase inhibitor used in epilepsy treatment, reduces intracellular protons. This shifts voltage-dependent activation of HCN channels positively, similarly to intracellular acidification.
Extracellular Cesium Ions: these bind to HCN and act as a reversible blocker [56].
Interacting proteins [2295]
- Filamin A: interacts with HCN1 but not HCN2 or HCN4. This interaction hyperpolarizes HCN1 activation, reduces activation and deactivation kinetics, and leads to clustering of HCN1 channels at the membrane surface.
- Tamalin, S-SCAM, and Mint2 Scaffold Proteins: These interactions likely play a role in channel trafficking, distribution, and clustering.
- TRIP8b (Brain-Specific Cytoplasmic Protein): TRIP8b interacts with HCN channels in the mammalian brain, particularly HCN1. TRIP8b has multiple splice variants that differentially regulate HCN1 channel surface expression.
Neurotransmitters: [2295]
- Acetylcholine (ACh)
- Monoaminergic neurotransmitters (NE, serotonin, dopamine)
- Glutamate
- Purinergic neurotransmitters (ATP, adenosine)
- Nitric oxide (NO)
Neuropeptides [2295]
- Orexins (Orexin-A and Orexin-B)
- Opioid Peptides (Enkephalins, Endorphins, Dynorphins)
- Neuropeptide Y (NPY)
- Neurotensin (NT)
- Substance P (SP)
- Cortistatin (CST)
- Vasoactive Intestinal Polypeptide (VIP) and Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)
- Corticotrophin-Releasing Factor (CRF)
- Angiotensin II (Ang II)
For additional HCN interacting compound resources, known and predicted animal toxin interactions with HCN
References
Stieber J
et al.
Functional expression of the human HCN3 channel.
J. Biol. Chem.,
2005
Oct
14
, 280 (34635-43).
Kole MH
et al.
Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output.
J. Neurosci.,
2006
Feb
8
, 26 (1677-87).
Nusser Z
Variability in the subcellular distribution of ion channels increases neuronal diversity.
Trends Neurosci.,
2009
May
, 32 (267-74).
Shah MM
et al.
Seizure-induced plasticity of h channels in entorhinal cortical layer III pyramidal neurons.
Neuron,
2004
Oct
28
, 44 (495-508).
Notomi T
et al.
Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain.
J. Comp. Neurol.,
2004
Apr
5
, 471 (241-76).
Ballo AW
et al.
Dopamine modulates Ih in a motor axon.
J. Neurosci.,
2010
Jun
23
, 30 (8425-34).
Monteggia LM
et al.
Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain.
Brain Res. Mol. Brain Res.,
2000
Sep
30
, 81 (129-39).
Moosmang S
et al.
Differential distribution of four hyperpolarization-activated cation channels in mouse brain.
Biol. Chem.,
1999 Jul-Aug
, 380 (975-80).
Giorgetti A
et al.
A homology model of the pore region of HCN channels.
Biophys. J.,
2005
Aug
, 89 (932-44).
Biel M
et al.
Hyperpolarization-activated cation channels: from genes to function.
Physiol. Rev.,
2009
Jul
, 89 (847-85).
Benarroch EE
HCN channels: function and clinical implications.
Neurology,
2013
Jan
15
, 80 (304-10).
Davoine F
et al.
Response to coincident inputs in electrically coupled primary afferents is heterogeneous and is enhanced by H-current (IH) modulation.
J. Neurophysiol.,
2019
Jul
01
, 122 (151-175).
Rivolta I
et al.
Cardiac and neuronal HCN channelopathies.
Pflugers Arch, 2020Jul, 472 (931-951).
Sartiani L
et al.
The Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels: from Biophysics to Pharmacology of a Unique Family of Ion Channels.
Pharmacol Rev, 2017Oct, 69 (354-395).
Zagotta WN
et al.
Structural basis for modulation and agonist specificity of HCN pacemaker channels.
Nature,
2003
Sep
11
, 425 (200-5).
He C
et al.
Neurophysiology of HCN channels: From cellular functions to multiple regulations.
Prog. Neurobiol.,
2014
Jan
, 112 (1-23).
Much B
et al.
Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels.
J. Biol. Chem.,
2003
Oct
31
, 278 (43781-6).
Poolos NP
et al.
Modulation of h-channels in hippocampal pyramidal neurons by p38 mitogen-activated protein kinase.
J. Neurosci.,
2006
Jul
26
, 26 (7995-8003).
Itoh M
et al.
The hyperpolarization-activated cyclic nucleotide-gated (HCN) channels contain multiple S-palmitoylation sites.
J Physiol Sci, 2016May, 66 (241-8).
Lee CH
et al.
Structures of the Human HCN1 Hyperpolarization-Activated Channel.
Cell, 2017Jan12, 168 (111-120.e11).
Wainger BJ
et al.
Molecular mechanism of cAMP modulation of HCN pacemaker channels.
Nature,
2001
Jun
14
, 411 (805-10).
Day M
et al.
Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels.
J. Neurosci.,
2005
Sep
21
, 25 (8776-87).
Zong X
et al.
Regulation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel activity by cCMP.
J. Biol. Chem.,
2012
Aug
3
, 287 (26506-12).
Ulens C
et al.
Regulation of hyperpolarization-activated HCN channels by cAMP through a gating switch in binding domain symmetry.
Neuron,
2003
Dec
4
, 40 (959-70).
Lolicato M
et al.
Tetramerization dynamics of C-terminal domain underlies isoform-specific cAMP gating in hyperpolarization-activated cyclic nucleotide-gated channels.
J. Biol. Chem.,
2011
Dec
30
, 286 (44811-20).
Stieber J
et al.
Bradycardic and proarrhythmic properties of sinus node inhibitors.
Mol Pharmacol, 2006Apr, 69 (1328-37).
Contributors: Rajnish Ranjan, Michael Schartner
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/206/ , accessed on 2026 Feb 21