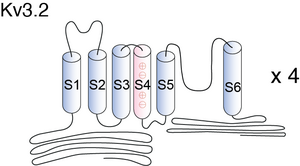
Kv3.2
Description: potassium voltage gated channel, Shaw-related subfamily, member 2 Gene: Kcnc2 Alias: Kv3.2, kcnc2, KShIIIA
Kv3.2, encoded by the gene KCNC2, is a voltage-gated potassium channel. Kv3.2 plays an essential role in regulating neuronal firing.
Experimental data
Rat Kv3.2 gene in CHO host cells datasheet |
||
|
Click for details 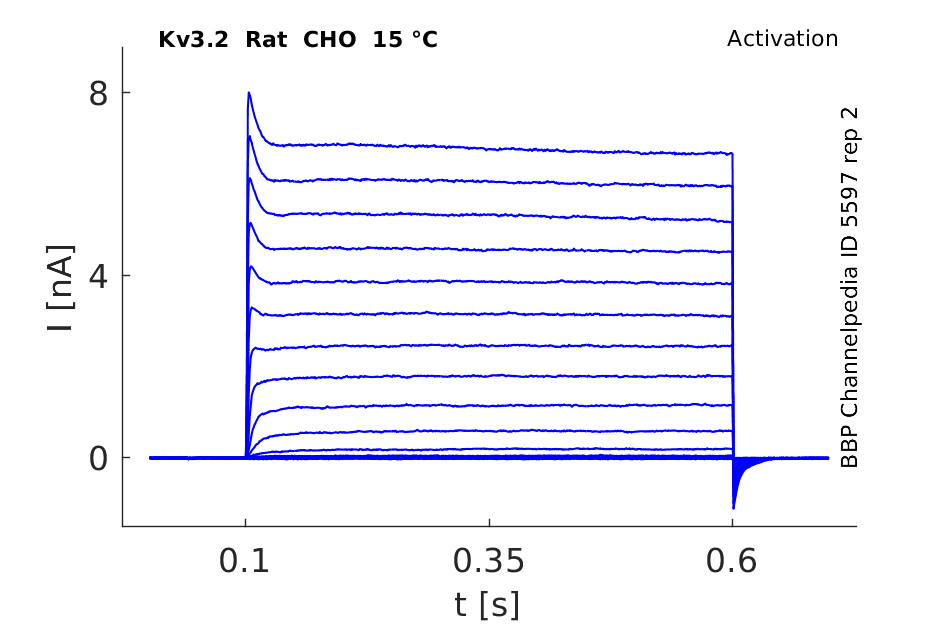
15 °Cshow 50 cells |
Click for details 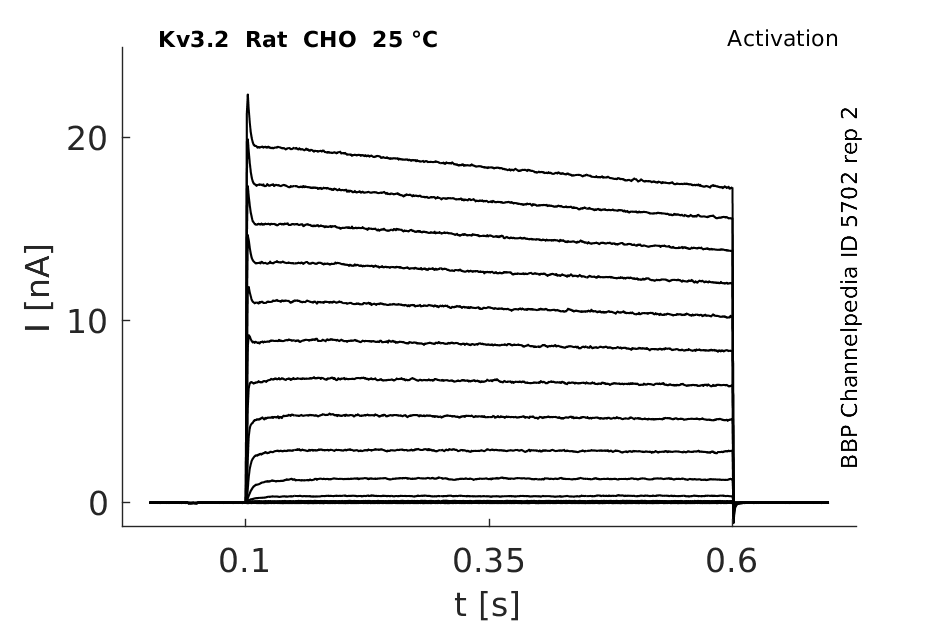
25 °Cshow 108 cells |
Click for details 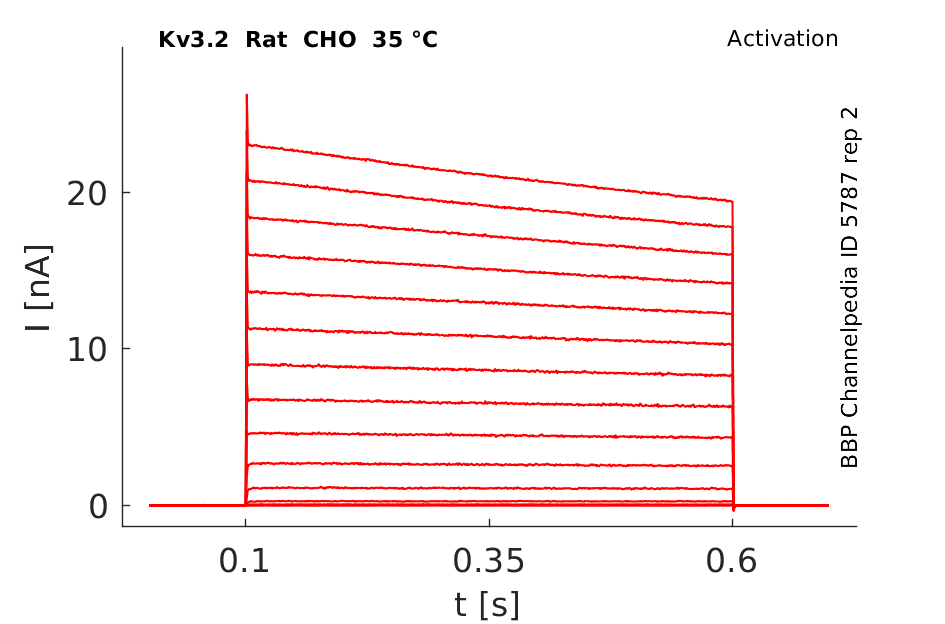
35 °Cshow 42 cells |
Gene
A number of transcript variant have been identified though only the functional consequences of these variants have not been extensively elucidated (see Expression and Distribution) [1547]
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_139136.4 | 3656 | |
| Mouse | NM_001359752.1 | 5063 | |
| Rat | NM_139216.1 | 3064 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv3.2 Structure
Methodology for visual representation of structure available here
The number of charged residues in the S4 segment is an obvious difference in the structure between Kv3 and Shaker and its mammalian homologous channels. A valine residue in Kv3.2b channel replaces the lysine at the cytoplasmic end of S4 (K380) in Shaker. However, the effect of mutating lysine to valine at this residue on the Shaker gating current has not been tested. Exchange of the cytoplasmic half of S5 between Kv3.1 and Kv2.1 channels significantly affected the kinetics of deactivation and Ig,off, suggesting that S5 participates in the conformational rearrangements associated with the concerted step(s) in channel activation and early step(s) in deactivation, but no effect on I g,on was observed. Our data from Kv3.2b P468W showed little change in Ig,on [1551]
Kv3.2 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
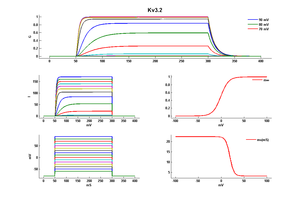
Kv3.2 Channel Activation
Both Kv3.2a or Kv3.1b currents start activating when the membrane is depolarized to potentials more positive than −10 mV and rise relatively fast (as compared with other voltage-gated K+ channels), with a similar time course, to a maximum level that is maintained for the duration of the pulses used in this experiment [18]
Kv3.2 Channel Inactivation
A slow inactivation becomes evident with pulses of longer duration. An unusual feature of Kv3 currents is the fast rate of deactivation on repolarization. Kv3.1 currents also deactivate extremely fast in CHO cells under our recording conditions. Kv3.2 currents deactivate at rates somewhat slower than Kv3.1 but still significantly faster than K+ currents from channels of other subfamilies [18]
Kv3.2 Expressed in CHO cells
Kv3.2 currents elicited in response to voltage clamp steps from -50 to +40 mV delivered every 15 s from a holding potential of -90 mV in 10 mV increments. The results were also very similar to what has been seen in X.oocytes [1550]
Comparison of Kinetics to other Kv Channels
The rate of rise of Kv3.1−Kv3.3 currents is relatively fast; faster than many other voltage-gated K+ channels (e.g., Kv2.x; Kv1.2) but slower than that of other voltage-gated K+channels such as several Kv1 channels like Kv1.1, Kv1.4 and Kv1.5. Kv3.4 currents rise faster than Kv3.1−Kv3.3 currents. In contrast to other delayed rectifiers, Kv3.1 and Kv3.2 currents are not significantly inactivated by depolarizing prepulses and do not show cumulative inactivation. Records of Kv3.1 and Kv3.2 currents in mammalian cells (but not in oocytes)often show an initial fast transient component. This is usually very small (<5% of the current)and is seen mainly in response to large depolarizing pulses (to >+30 mV). The origin of this component is not clear yet. In our experience its presence and magnitude are very variable; it is more readily seen in HEK293T than in CHO cells; and it is bigger in cells expressing large currents. Critz et al.27 have suggested that it might be associated with K+ accumulation in the extracellular space.[290]
Single Channel Conductance
Single channel recordings of Kv3.2-transfected CHO cells were performed in the inside-out configuration of the patch clamp technique. Channels were recorded during large depolarizations, when the probability of channel opening is high [1550]

Biophysics
Model Kv3.2 (ID=39)
| Animal | CH | |
| CellType | CHO | |
| Age | 0 Days | |
| Temperature | 22.0°C | |
| Reversal | -80.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [18] R Hernandez-Pineda et. al; J. Neurophysiol. 1999 Sep | |
| mpower | 2.0 | |
| m Inf | 1/(1+exp((v- -0.373267)/-8.568187)) | |
| m Tau | 3.241643 +( 19.106496 / (1 + exp((v - 19.220623)/4.451533))) | |
Distribution patterns of Kv3.1b and Kv3.2 in human tissue
Significant, albeit low, levels of Kv3.1b immunoreactivity were detected in the human neocortex and cerebellum, specifically in DLPFC, PC and OC, but not in subcortical grey matter regions, that is, there were undetectable levels in CN, NAc, Thal and Hipp. These outcomes confirm a limited distribution of low levels of Kv3.1b protein in normal human neocortex. In contrast, high levels of Kv3.2 immunoreactivity were found broadly distributed through cortical and subcortical brain, including DLPFC, Hipp, CN, NAc and Thal [1546]
Amacrine Cells
With the exception of a very few ganglion cells, starburst amacrine cells were found to uniquely express Kv3.1 and Kv3.2 protein in the mouse retina [1552]
Kv3.1 and Kv3.2 are highly enriched in neurons that fire at high frequencies, such as fast spiking interneurons of the cortex and hippocampus [482], and neurons in the globus pallidus [302].
Kv3.2 was found in basolateral amygdalar interneurons such as parvalbumin neurons and somatostatin+ interneurons. [317]
Alcohol Exposure affects Expression
a significant reduction of the expression of Kv3.2 and Kv3.4 subunits occurred in alcohol-treated animals, as compared with controls. The expression of the Kv3.4a splicing variant, which is thought to be critically involved in the high-frequency firing of some cortical interneurons, was also correspondingly reduced. The downregulation of Kv3.2 and Kv3.4a subunits represented a long-lasting effect of alcohol exposure, since it was also observed in P24 animals. The expression of both Kv3.1 and Kv3.3 channels appeared to be not significantly affected by alcohol exposure [1547]
Activity Deprivation on Expression
We now describe the role of neuronal activity and neurotrophins for Kv3.1b/3.2 expression using organotypic cultures of rat visual cortex as model system. Chronic activity deprivation from 2 days in vitro (DIV) prevented the postnatal developmental increase of Kv3.2, but not Kv3.1b mRNA expression. However, chronic activity deprivation failed to alter Kv3.1b and marginally delayed Kv3.2 protein expression [1805]
Kv3.2 Distribution in Neuron
Kv3.2 was determined to be expressed primarily in clusters on the surface of interneuron somata and proximal dendrites of positive cells. Although a large proportion of the Kv3.2 immuno- stain was also found throughout the neuropil, we were unable to determine the exact structures (e.g., dendrites, axons) associated with this immunostaining [1548]
In most Kv3.1- and Kv3.2-expressing neurons, the protein is localized in somatic and axonal membranes, with little expression in dendrites other than their initial segments. It is clear that the axon- less starburst cells represent an unusual distribution of Kv3 subunits within the soma and dendritic processes. In this way, starburst cells resemble the mitral cells of the olfactory bulb in which Kv3.1 proteins are also prominently expressed throughout the dendritic arbor [1552]
Seizure Susceptibility
Pharmacological or genetic disruption of Kv3 currents leads to impaired fast spiking in inhibitory neurons and increased seizure susceptibility [484].
Action potential repolarization
The electrophysiological properties of Kv3 channels suggest a specific role in action potential repolarization. Given their voltage dependence, it is unlikely that Kv3 channels will be significantly activated other than during action potentials, and only once these have reached their peak. These properties suggest that, when present in sufficient amounts, Kv3 channels can facilitate action potential repolarization and dictate action potential duration, without compromising action potential threshold, rise time or magnitude, and, more importantly, without contributing current that would increase the refractory period duration. This is in contrast to voltage-gated K+ channels that activate significantly at more negative potentials and deactivate more slowly [302]
Selective modulation of Kv3.2 channels in OA interneurones by cAMP is likely to be an important factor regulating the activity of dendritic inhibitory cells in principal neurone-interneurone microcircuits [1549]
Stichodactyla helianthus peptide
Stichodactyla helianthus peptide is a potent inhibitor of Kv3.2 channels and may serve as a useful pharmacological probe for studying these channels in native preparations.[19]
Tetraethylammonium
Tetraethylammonium concentrations (1 mM) block only a few known K+channels including Kv3.1-Kv3.2. [483]
MinK, MiRP1 and MiRP2
Modulation by MinK and MinK-related peptides MiRP1 and MiRP2 is a general mechanism for slowing of Kv3.1 and Kv3.2 channel activation and deactivation and acceleration of inactivation, creating a functionally diverse range of channel complexes. [15]
Exposure to Ethanol
The main finding of the present study is represented by the demonstration that Kv3.2 and Kv3.4 subunit transcripts show a selective and lasting underexpression in the sensori-motor cortex of rats exposed to ethanol during the first postnatal week [1547]
TEA and 4-aminopyridine (4-AP)
At present there are no specific blockers for Kv3.1 and Kv3.2 channels. However, in Xenopus oocytes all Kv3 currents are blocked by low concentrations of TEA or 4-aminopyridine (4-AP). Kv3.1 and Kv3.2 currents in CHO cells, under our recording conditions, were also very sensitive to these channel blockers. The concentration of TEA used to isolate the current in pallidal neurons (1 mM) blocks >80% of Kv3.1 and Kv3.2 currents in heterologous expression systems. [18]
Blood Depressing Toxins
BDS is not selective for Kv3.4 but markedly inhibits current through Kv3.1 and Kv3.2. Inhibition comes about not by “pore block” but by striking modification of Kv3 gating kinetics and voltage dependence. Activation and inactivation kinetics are slowed by BDS-I and BDS-II, and V1/2 for activation is shifted to more positive voltages. Alanine substitution mutagenesis around the S3b and S4 segments of Kv3.2 reveals that BDS acts via voltage-sensing domains, and, consistent with this, ON gating currents from nonconducting Kv3.2 are markedly inhibited [23]
Nitric Oxide (NO)
Kv3.1 and Kv3.2 currents are potentially suppressed by D-NONOate and other NO donors. The effects of NO on these currents are mediated by the activation of guanylyl cyclase (GC), since they are prevented by Methylene Blue, an inhibitor of GC, and by ODQ, a specific inhibitor of the soluble form of GC. Moreover, application of 8-Br-cGMP, a permeant analogue of cGMP, also blocked Kv3.1 and Kv3.2 currents. 4.KT5283, a cGMP-dependent protein kinase (PKG) blocker, prevented the inhibition of Kv3.1 and Kv3.2 currents by D-NONOate and 8-Br-cGMP [1550]
Negatively modulated by Mg2+
Kv3.1 and Kv3.2 channels are negatively modulated by intracellular Mg2+ in a voltage-dependent manner, producing a negative slope conductance in the conductance–voltage curve d and e. Variations in the conductance–voltage relationship between different studies on mammalian cells might be associated with differences in intracellular Mg2+ concentrations: lower Mg2+ concentrations causing an apparent rightward shift in the voltage dependence [302]
H2 Histamine
H2 receptor activation negatively modulates outward currents through Kv3.2-containing potassium channels by a mechanism involving PKA phosphorylation in inhibitory interneurons. PKA phosphorylation of Kv3.2 lowered the maximum firing frequency of inhibitory neurons, which in turn negatively modulated high-frequency population oscillations recorded in principal cell layers. All these effects were absent in a Kv3.2 knockout mouse [1806]
References
Lewis A
et al.
MinK, MiRP1, and MiRP2 diversify Kv3.1 and Kv3.2 potassium channel gating.
J. Biol. Chem.,
2004
Feb
27
, 279 (7884-92).
Hernandez-Pineda R
et al.
Kv3.1-Kv3.2 channels underlie a high-voltage-activating component of the delayed rectifier K+ current in projecting neurons from the globus pallidus.
J. Neurophysiol.,
1999
Sep
, 82 (1512-28).
Yan L
et al.
Stichodactyla helianthus peptide, a pharmacological tool for studying Kv3.2 channels.
Mol. Pharmacol.,
2005
May
, 67 (1513-21).
Yeung SY
et al.
Modulation of Kv3 subfamily potassium currents by the sea anemone toxin BDS: significance for CNS and biophysical studies.
J. Neurosci.,
2005
Sep
21
, 25 (8735-45).
Chow A
et al.
K(+) channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons.
J. Neurosci.,
1999
Nov
1
, 19 (9332-45).
Rudy B
et al.
Contributions of Kv3 channels to neuronal excitability.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (304-43).
Rudy B
et al.
Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing.
Trends Neurosci.,
2001
Sep
, 24 (517-26).
Gan L
et al.
When, where, and how much? Expression of the Kv3.1 potassium channel in high-frequency firing neurons.
J. Neurobiol.,
1998
Oct
, 37 (69-79).
McDonald AJ
et al.
Differential expression of Kv3.1b and Kv3.2 potassium channel subunits in interneurons of the basolateral amygdala.
Neuroscience,
2006
, 138 (537-47).
Erisir A
et al.
Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons.
J. Neurophysiol.,
1999
Nov
, 82 (2476-89).
Lau D
et al.
Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins.
J. Neurosci.,
2000
Dec
15
, 20 (9071-85).
Coetzee WA
et al.
Molecular diversity of K+ channels.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (233-85).
Yanagi M
et al.
Kv3.1-containing K(+) channels are reduced in untreated schizophrenia and normalized with antipsychotic drugs.
Mol. Psychiatry,
2013
Apr
30
, ().
Tavian D
et al.
Selective underexpression of Kv3.2 and Kv3.4 channels in the cortex of rats exposed to ethanol during early postnatal life.
Neurol. Sci.,
2011
Aug
, 32 (571-7).
Tansey EP
et al.
Developmental expression of potassium-channel subunit Kv3.2 within subpopulations of mouse hippocampal inhibitory interneurons.
,
2002
, 12 (137-48).
Lien CC
et al.
Gating, modulation and subunit composition of voltage-gated K(+) channels in dendritic inhibitory interneurones of rat hippocampus.
J. Physiol. (Lond.),
2002
Jan
15
, 538 (405-19).
Moreno H
et al.
Modulation of Kv3 potassium channels expressed in CHO cells by a nitric oxide-activated phosphatase.
J. Physiol. (Lond.),
2001
Feb
1
, 530 (345-58).
Wang Z
et al.
Gating currents from a Kv3 subfamily potassium channel: charge movement and modification by BDS-II toxin.
J. Physiol. (Lond.),
2007
Nov
1
, 584 (755-67).
Ozaita A
et al.
A unique role for Kv3 voltage-gated potassium channels in starburst amacrine cell signaling in mouse retina.
J. Neurosci.,
2004
Aug
18
, 24 (7335-43).
Grabert J
et al.
Neuronal activity and TrkB ligands influence Kv3.1b and Kv3.2 expression in developing cortical interneurons.
Neuroscience,
2008
Oct
15
, 156 (618-29).
Atzori M
et al.
H2 histamine receptor-phosphorylation of Kv3.2 modulates interneuron fast spiking.
Nat. Neurosci.,
2000
Aug
, 3 (791-8).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/12/ , accessed on 2026 Feb 24