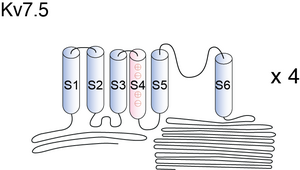
Kv7.5
Description: potassium voltage-gated channel, KQT-like subfamily, member 5 Gene: Kcnq5 Alias: Kv7.5, KCNQ5
Kv7.5, encoded by the gene KCNQ5, is a member of the potassium voltage-gated channel KQT-like subfamily. Kv7.5 is differentially expressed in subregions of the brain and in skeletal muscle. The protein encoded by this gene yields currents (M current) that activate slowly with depolarization and can form heteromeric channels with the protein encoded by the KCNQ3 gene. NCBI
Experimental data
Rat Kv7.5 gene in CHO host cells datasheet |
||
|
Click for details 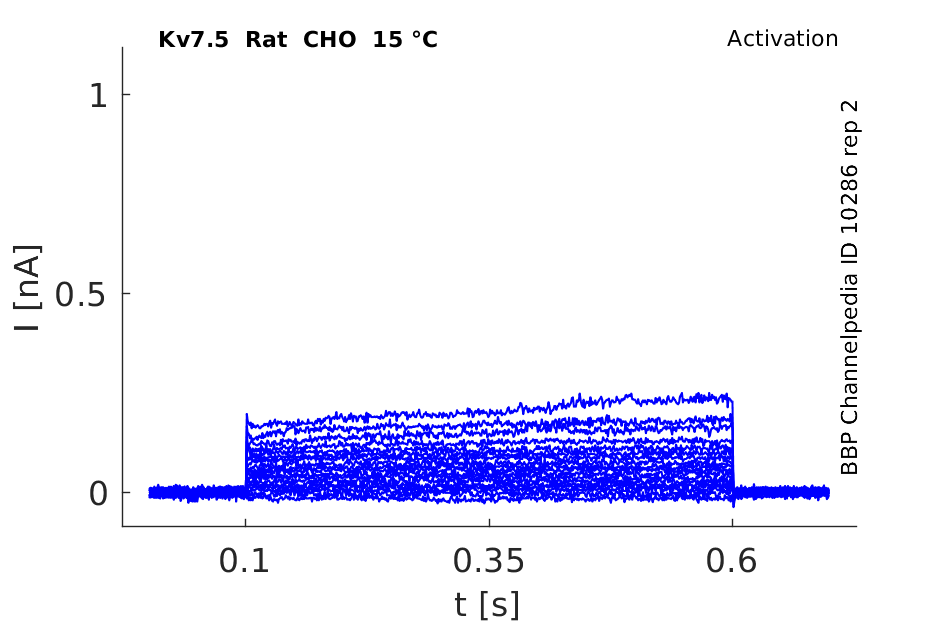
15 °Cshow 50 cells |
Click for details 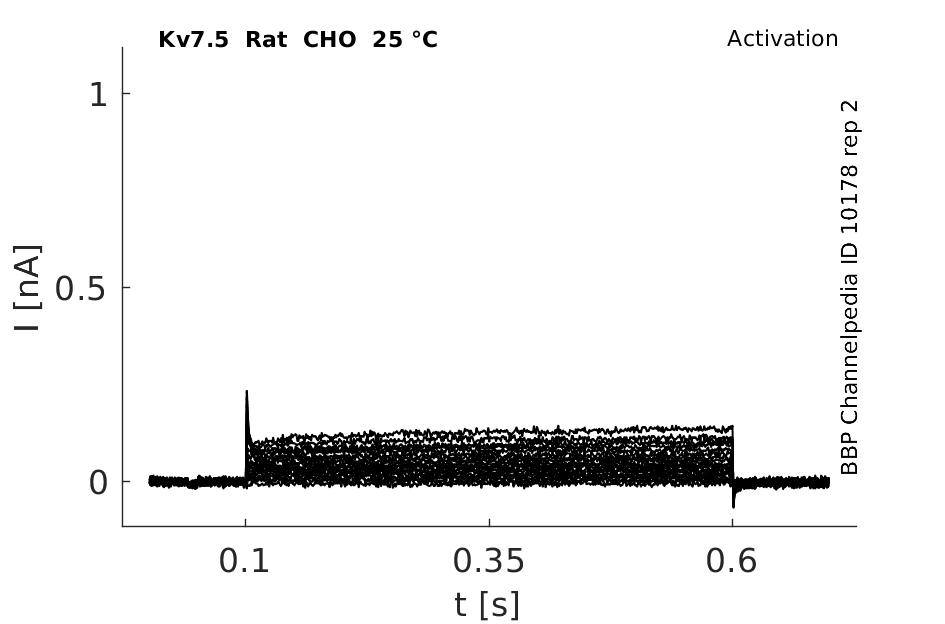
25 °Cshow 65 cells |
Click for details 
35 °Cshow 77 cells |
Rat Kv7.5 gene in HEK host cells |
||
|
Click for details 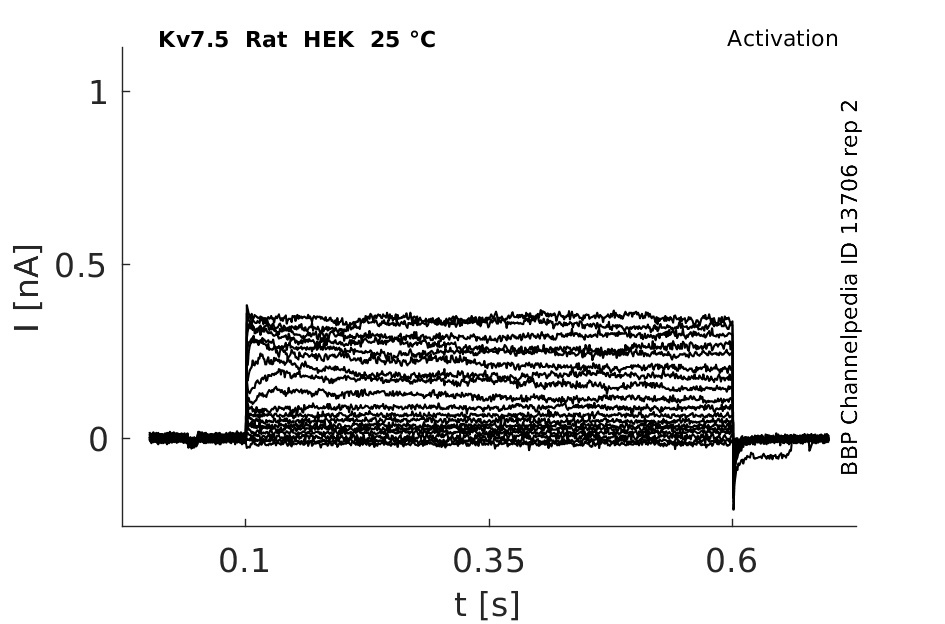
25 °Cshow 48 cells |
||
Rat Kv7.5 gene in CV1 host cells |
||
|
Click for details 
25 °Cshow 64 cells |
||
Gene
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_019842.4 | 6364 | |
| Mouse | NM_001160139.1 | 6992 | |
| Rat | NM_001134643.2 | 3807 |
Multiple transcript variants encoding different isoforms have been found for this gene. NCBI
Isoforms
Post-Translational Modifications
Visual Representation of Kv7.5 Structure
Methodology for visual representation of structure available here
KCNQ channels have a membrane topology similar to the Kv channels, comprising six transmembrane-spanning segments (S1 –S6) with a typical S4 domain, which is the voltage sensor, a pore loop linking S5 and S6, and intracellular N and C termini. (Dupuis [138])
Kv7.5 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Human KCNQ5/Q3 in CHO cell Kinetics

KCNQ5 Kinetics expressed in X oocytes
Currents activated very slowly (Fig.3 A) and were not fully activated even after 3-s test pulses. At lower step potentials, KCNQ5 currents showed a delay in activation, similar to KCNQ1 currents (32). In most cells (in 23 out of 28 oocytes) activation traces above +20 mV displayed a “crossover” phenomenon, which was observed independently of current amplitudes. Activation of KCNQ5 currents was generally slower than that of other KCNQ currents. Two components of deactivation, with time constants of 51 ± 2 and 281 ± 24 ms, were observed at a repolarizing voltage of −100 mV, following a 3-s depolarizing step to +40 mV (Table I). KCNQ1 tail currents display a characteristic “hook” indicative of recovery from inactivation (32, 33). We did not observe such a feature for KCNQ5 currents at voltages between −100 and +40 mV [140]
KCNQ5 yields currents that activate slowly with depolarization. [724] (Depuis [138])
Kv7.5 expression in the Brain
This includes prominent signals in the cortex, the occipital pole, frontal and temporal lobes, the caudate putamen, and the hippocampus [724] Kv7.5 is highly expressed in skeletal muscle [715] as well as certain regions of the brain [724]. Murine vascular smooth muscle cells only express a truncated form of KCNQ5. [139]
Localization of KCNQ5 in the normal and epileptic human temporal neocortex and hippocampal formation [1822]
Kv7.5 expression in body
KCNQ5 is expressed in skeletal muscle and shows widespread expression in the central nervous system. The expression of KCNQ5 is in many brain regions overlapping with the expression of KCNQ2 and KCNQ3 (Lerche et al., 2000 [140]; Schroeder et al., 2000 [724]).
KCNQ5 gene messages were present abundantly in murine vasculature (Ohya [1092], Yeung [1093]).
Kv7.5 Distribution in Neuron
Of its five known subunits, KCNQ5/Kv7.5 is extensively expressed in the central nervous system and it contributes to the generation of M-currents. The distribution of KCNQ5 was analyzed in auditory nuclei of the rat brainstem by high-resolution immunocytochemistry. Double labeling with anti-KCNQ5 antibodies and anti-synaptophysin or anti-syntaxin, which mark synaptic endings, or anti-microtubule-associated protein 2 (MAP2) antibodies, which mark dendrites, were used to analyze the subcellular distribution of KCNQ5 in neurons in the cochlear nucleus, superior olivary complex, nuclei of the lateral lemniscus, and inferior colliculus [1821]
Myoblast Proliferation
Kv7.5 is involved in myoblast proliferation [714].
M current
the function of KCNQ5 in the brain remains unknown and no neurological disorders have been attributed to it. Given KCNQ5’s similar biophysical properties to other members of the KCNQ family, KCNQ5 may also contribute to M-currents [461]
The Kv7 family is unique in the sense that mutations in four of the five genes have been linked to human hereditary diseases [722], [464].
KCNQ5 channels control resting properties and release probability of a synapse
Unlike most KCNQ channels, which are activated only by depolarizing stimuli, the presynaptic channels began to activate just below the resting potential. As a result, blockers and activators of KCNQ5 depolarized or hyperpolarized nerve terminals, respectively, markedly altering resting conductance. Moreover, the background conductance set by KCNQ5 channels, together with Na(+) and hyperpolarization-activated and cyclic nucleotide-gated (HCN) channels, determined the size and time course of the response to sub threshold stimuli [1820]
Mediates after-hyperpolarization
the function of KCNQ5 (Kv7.5), which also displays widespread expression in the brain, is entirely unknown. Here, we developed mice that carry a dominant negative mutation in the KCNQ5 pore to probe whether it has a similar function as other KCNQ channels. In the CA3 area of hippocampus, a region that highly expresses KCNQ5 channels, the medium and slow afterhyperpolarization currents are significantly reduced. In contrast, neither current is affected in the CA1 area of the hippocampus, a region with low KCNQ5 expression. Our results demonstrate that KCNQ5 channels contribute to the afterhyperpolarization currents in hippocampus in a cell type-specific manner [1746]
Myogenicity and Vasodilation in the Brain
In cerebral arteries, Kv7.4 and Kv7.5 proteins exist predominantly as a functional heterotetramer, which regulates intrinsic myogenicity and vasodilation attributed to CGRP. Surprisingly, unlike systemic arteries, Kv7 activity in MCAs is not affected by the development of hypertension, and CGRP (calcitonin gene-related peptide)-mediated vasodilation is well maintained. As such, cerebrovascular Kv7 channels could be amenable for therapeutic targeting in conditions such as cerebral vasospasm [1824]
KCNE channel alters KCNQ5 channel gating
The proteins KCNE1 and KCNE3 alter the gating of the Kv7.5 channel in Xenopus oocytes and HEK-293 cells. While KCNE1 slows activation and inhibits inward rectification, KCNE3 inhibits the current amplitude and differentially affects activation. Furthermore, KCNE1 increases Kv7.5 currents, when co-expressed in HEK cells. [710]
Calmodulin a Calcium sensor
Compared with other Kv channels, KCNQ channels have an extended intracellular carboxyl terminus that seems to be the target of many modulatory signals. Examples are modulation by Ca2+ [716], using calmodulin as the channel Ca2+ sensor [717], and regulation by plasma membrane phosphoinositides [718], [719], [720] perhaps in concert with protein kinase C [721].
Cell volume and Zinc
Changes in cell volume, extracellular Zn2+, acidification, and muscarinic receptor activation modulate Kv7.5 [723],[724].
KCNQ3
KCNQ5 forms heteromeric channels with KCNQ3, but not with KCNQ1, KCNQ2 or KCNQ4 (Lerche et al., 2000 [140]; Schroeder et al., 2000 [724]). KCNQ5 is inhibited by the M1 muscarinic receptor activation. (Depuis [138])
KCNQ2
Co-injection of KCNQ5 with the dominant negative mutant KCNQ2(G279S) (7) or of KCNQ2 with the equivalent mutant KCNQ5(G278S) leads to a roughly 50% reduction in current amplitude, which is consistent with a lack of interaction since only 50% of WT cRNA was injected [724]
BMS-204352, a KCNQ5 activator
Anti-ischemic compound, BMS-204352, strongly activates the voltage-gated K+ channel KCNQ5 in a concentration-dependent manner with an EC50 of 2.4 micro mol. Retigabine induced a smaller, yet qualitatively similar effect on KCNQ5. Furthermore, BMS-204352 (10 mM) did not significantly shift the KCNQ5 activation curves, as observed for the other KCNQ channels. The M-current blockers, linopirdine and XE991, inhibited the activation of the KCNQ5 channel induced by the BMS-204352. (Depuis [138])
Effect of Retigabine on KCNQ3/5 heteromeric channel
M1 Muscarinic Inhibition
Currents expressed from KCNQ5 have voltage dependences and inhibitor sensitivities in common with M-currents. They are also inhibited by M1 muscarinic receptor activation
Linopirdine
Linopirdine has inhibitory effects on KCNQ5 [724]
TEA
Kv7.5 is poorly inhibited by the common potassium inhibitor TEA [724]
References
Dupuis DS
et al.
Activation of KCNQ5 channels stably expressed in HEK293 cells by BMS-204352.
Eur. J. Pharmacol.,
2002
Feb
22
, 437 (129-37).
Yeung SY
et al.
Expression profile and characterisation of a truncated KCNQ5 splice variant.
Biochem. Biophys. Res. Commun.,
2008
Jul
11
, 371 (741-6).
Lerche C
et al.
Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity.
J. Biol. Chem.,
2000
Jul
21
, 275 (22395-400).
Jentsch TJ
Neuronal KCNQ potassium channels: physiology and role in disease.
Nat. Rev. Neurosci.,
2000
Oct
, 1 (21-30).
Bailey SD
et al.
Variation at the NFATC2 locus increases the risk of thiazolidinedione-induced edema in the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) study.
Diabetes Care,
2010
Oct
, 33 (2250-3).
Talmud PJ
et al.
Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip.
Am. J. Hum. Genet.,
2009
Nov
, 85 (628-42).
Gutman GA
et al.
International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels.
Pharmacol. Rev.,
2005
Dec
, 57 (473-508).
Wang HS
et al.
KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel.
Science,
1998
Dec
4
, 282 (1890-3).
Rose JE
et al.
Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score.
Mol. Med.,
2010 Jul-Aug
, 16 (247-53).
Roura-Ferrer M
et al.
Functional implications of KCNE subunit expression for the Kv7.5 (KCNQ5) channel.
Cell. Physiol. Biochem.,
2009
, 24 (325-34).
Bal M
et al.
Homomeric and heteromeric assembly of KCNQ (Kv7) K+ channels assayed by total internal reflection fluorescence/fluorescence resonance energy transfer and patch clamp analysis.
J. Biol. Chem.,
2008
Nov
7
, 283 (30668-76).
Jensen HS
et al.
Inactivation as a new regulatory mechanism for neuronal Kv7 channels.
Biophys. J.,
2007
Apr
15
, 92 (2747-56).
Li Y
et al.
Dual phosphorylations underlie modulation of unitary KCNQ K(+) channels by Src tyrosine kinase.
J. Biol. Chem.,
2004
Oct
29
, 279 (45399-407).
Roura-Ferrer M
et al.
Skeletal muscle Kv7 (KCNQ) channels in myoblast differentiation and proliferation.
Biochem. Biophys. Res. Commun.,
2008
May
16
, 369 (1094-7).
Mackie AR
et al.
Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance.
J. Pharmacol. Exp. Ther.,
2008
May
, 325 (475-83).
Selyanko AA
et al.
Intracellular calcium directly inhibits potassium M channels in excised membrane patches from rat sympathetic neurons.
Neuron,
1996
Jan
, 16 (151-62).
Gamper N
et al.
Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels.
J. Gen. Physiol.,
2003
Jul
, 122 (17-31).
Suh BC
et al.
Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis.
Neuron,
2002
Aug
1
, 35 (507-20).
Suh BC
et al.
Regulation of KCNQ2/KCNQ3 current by G protein cycling: the kinetics of receptor-mediated signaling by Gq.
J. Gen. Physiol.,
2004
Jun
, 123 (663-83).
Zhang H
et al.
PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents.
Neuron,
2003
Mar
27
, 37 (963-75).
Hoshi N
et al.
AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists.
Nat. Neurosci.,
2003
Jun
, 6 (564-71).
Robbins J
KCNQ potassium channels: physiology, pathophysiology, and pharmacology.
Pharmacol. Ther.,
2001
Apr
, 90 (1-19).
Jensen HS
et al.
The KCNQ5 potassium channel from mouse: a broadly expressed M-current like potassium channel modulated by zinc, pH, and volume changes.
Brain Res. Mol. Brain Res.,
2005
Sep
13
, 139 (52-62).
Schroeder BC
et al.
KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents.
J. Biol. Chem.,
2000
Aug
4
, 275 (24089-95).
Ohya S
et al.
Molecular variants of KCNQ channels expressed in murine portal vein myocytes: a role in delayed rectifier current.
Circ. Res.,
2003
May
16
, 92 (1016-23).
Yeung SY
et al.
Molecular expression and pharmacological identification of a role for K(v)7 channels in murine vascular reactivity.
Br. J. Pharmacol.,
2007
Jul
, 151 (758-70).
Wickenden AD
et al.
Characterization of KCNQ5/Q3 potassium channels expressed in mammalian cells.
Br. J. Pharmacol.,
2001
Jan
, 132 (381-4).
Tzingounis AV
et al.
The KCNQ5 potassium channel mediates a component of the afterhyperpolarization current in mouse hippocampus.
Proc. Natl. Acad. Sci. U.S.A.,
2010
Jun
1
, 107 (10232-7).
Huang H
et al.
KCNQ5 channels control resting properties and release probability of a synapse.
Nat. Neurosci.,
2011
, 14 (840-7).
Caminos E
et al.
The potassium channel KCNQ5/Kv7.5 is localized in synaptic endings of auditory brainstem nuclei of the rat.
J. Comp. Neurol.,
2007
Dec
1
, 505 (363-78).
Yus-Nájera E
et al.
Localization of KCNQ5 in the normal and epileptic human temporal neocortex and hippocampal formation.
Neuroscience,
2003
, 120 (353-64).
Chadha PS
et al.
Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity.
Arterioscler. Thromb. Vasc. Biol.,
2014
Apr
, 34 (887-93).
Contributors: Rajnish Ranjan, Michael Schartner, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/27/ , accessed on 2026 Feb 23