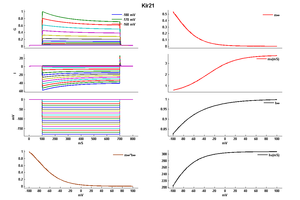
Kir2.1
Description: potassium inwardly-rectifying channel, subfamily J, member 2 Gene: Kcnj2 Alias: Kir2.1, IRK1, LQT7, SQT3, HHIRK1, HHBIRK1, KCNJ2
KCNJ2 (also known as IRK1; LQT7; SQT3; ATFB9; HHIRK1; KIR2.1; HHBIRK1) encodes member 2 of subfamily J of potassium inwardly-rectifying channels, which is called Kir2.1. This channel has a greater tendency to allow potassium to flow into a cell rather than out of a cell, probably participates in establishing action potential waveform and excitability of neuronal and muscle tissues. Mutations in this gene have been associated with Andersen syndrome, which is characterized by periodic paralysis, cardiac arrhythmias, and dysmorphic features. http://www.ncbi.nlm.nih.gov/gene/3759
Kir2.1, along with Kir2.2 and Kir2.3, is thought to underlie the background inward rectifier K+ current I K,ACh (Liu et al. 2001; [909] Zaritsky et al. 2001 [910]; Zobel et al. 2003 [911]). Kir2.1 channel underlies the cardiac current I K,1. Makary [182]
Experimental data
Rat Kir2.1 gene in CHO host cells |
||
|
Click for details 
25 °Cshow 48 cells |
Click for details 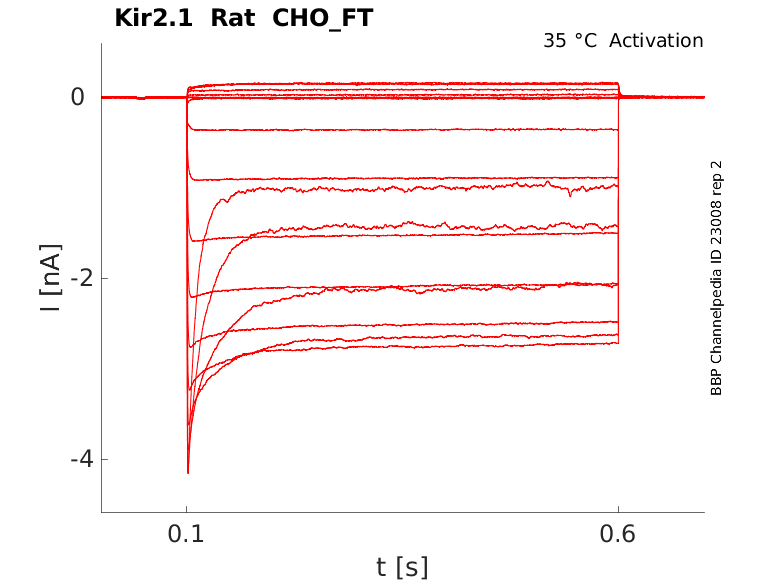
35 °Cshow 11 cells |
|
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_000891.3 | 5391 | |
| Mouse | NM_008425.4 | 5468 | |
| Rat | NM_017296.1 | 1284 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Cytoplasmic pore region of Kir2.1

In Kir2.1, a number of residues within the pore lining second transmembrane domain and proximal C terminus have been shown to be important for inward rectification (Lu & MacKinnon, 1994 [912]; Stanfield et al. 1994 [913]; Yang et al. 1995 [919]; Kubo & Murata, 2001 [914]; Fujiwara & Kubo, 2002 [915]). Recent evidence suggests that these may not be the site of block, but instead shuttle the polyamines to their eventual binding site deeper within the pore (Kubo & Murata, 2001 [914]; Guo et al. 2003 [916]; Xie et al. 2003 [917]; Chang et al. 2003 [918]).
Kir2.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Single Channel Conductance of Kir2.1

Rat Kir2.1 Skeletal Muscle Current Expressed in CHO

Mouse Kir2.1 Kinetics in CHO cells
Compared to Kir3
Kir3.1/Kir3.4 exhibits weaker inward rectification than Kir2.1. Makary [182]
Markov Model of Kir2.1 interaction with PIP2
Model Kir21 (ID=44)
| Animal | Chinese Hamster | |
| CellType | CHO | |
| Age | 0 Days | |
| Temperature | 28.0°C | |
| Reversal | -70.6 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [182] Samy M Y Makary et. al; J. Physiol. (Lond.) 2005 Nov 1 | |
| mpower | 1.0 | |
| m Inf | 1 /(1+exp((v-(-96.48))/23.26)) | |
| m Tau | 3.7 +( -3.37 / (1 + exp((v - -32.9)/27.93))) | |
| hpower | 2.0 | |
| h Inf | 1 /(1+exp((v-(-168.28))/-44.13)) | |
| h Tau | 0.85 + (306.3 / (1 + exp((v - -118.29)/-27.23))) | |
Kir2.1 is an inwardly rectifying K+ channel, being expressed in the heart. Makary [182] More Kir2.1 in atrial myocytes compared with ventricular cells. Panama [183]
AP Repolarization
I K1 - a current for which Kir2.1 mediates - regulates the late phase of action potential (AP) repolarization and stabilizes the resting membrane potential. In most species, the inward current density of atrial I K1 is significantly smaller than that of ventricles (Dhamoon [920], Giles [921], Melnik [922]).
QT Syndrome/Autism/Epilepsy
Genetically induced dysfunctions of Kir2.1 channels: implications for short QT3 syndrome and autism-epilepsy phenotype [1864]
ERAD
altered Kir2.1 levels lead to human disease and Kir2.1 restores growth on low-potassium medium in yeast mutated for endogenous potassium channels. Using this system, first we find that Kir2.1 is targeted for endoplasmic reticulum–associated degradation (ERAD).
Cardiac Channel
There is an increasing body of evidence that heteromeric assembly of Kir2.1, Kir2.2 and Kir2.3 potassium channels is the molecular basis of cardiac IK1 current [1869]
Golgi Export
Here, we show that the potassium channel Kir2.1, mutations in which are associated with Andersen-Tawil syndrome, is selected as cargo into Golgi export carriers in an unusual signal-dependent manner. Unlike conventional trafficking signals, which are typically comprised of short linear peptide sequences, Golgi exit of Kir2.1 is dictated by residues that are embedded within the confluence of two separate domains. This signal patch forms a recognition site for interaction with the AP1 adaptor complex, thereby marking Kir2.1 for incorporation into clathrin-coated vesicles at the trans-Golgi
Caveolin-1 (cholesterol) suppresses Kir2.1
We whether Cav-1 regulates the function of Kir2.1 channels that play major roles in the regulation of membrane potential of numerous mammalian cells. Our earlier studies demonstrated that Kir2.1 channels are cholesterol sensitive. In this study, we show that Kir2.1 channels co-immunoprecipitate with Cav-1 and that co-expression of Kir2.1 channels with Cav-1 in HEK293 cells results in suppression of Kir2 current indicating that Cav-1 is a negative regulator of Kir2 function [1862]
Extracellular Spermine block
Kir3.1/Kir3.4 is more sensitive to extracellular spermine block than Kir2.1, and that intracellular and extracellular polyamines can permeate Kir3.1/Kir3.4, but not Kir2.1, to a limited extent. Makary [182]
Kir2.3
Inclusion of only one Kir2.3 subunit to a Kir2.1 channel led to an approximate threefold slowing of activation kinetics, with greater slowing on subsequent additions of Kir2.3 subunits. Panama [183]
P639Amiodarone and Dronedarone
P639Amiodarone and dronedarone inhibit inwardly rectifying Kir2.1 channels, but not Kir2.2 and Kir2.3 channels [1863]
chloroethylclonidine
Chloroethylclonidine (CEC). The degree of current inhibition by CEC was found to vary with the membrane potential (approximately 70% block at -50 mV c.f. approximately 10% block at -190 mV). The kinetics of this voltage dependence were further investigated using recombinant inward rectifier K+ channels (Kir2.1) expressed in the MEL cell line [1865]
Mutation increases binding affinity
We find that a single mutation of tertiapin-Q increases the binding affinity for Kir2.1 by 5 orders of magnitude (K(d) = 0.7 nM). This potent blocker of Kir2.1 may serve as a structural template from which potent compounds for the treatment of various diseases mediated by this channel subfamily, such as cardiac arrhythmia, can be developed.
Kir3.1/Kir3.4
We examined if the subunits belonging to different subfamilies Kir2 and Kir3 can co-assemble to form heteromultimers in heterologous expression systems. We observed co-immunoprecipitation of Kir2.1 and Kir3.1 as well as Kir2.1 and Kir3.4 in HEK293T cells. Furthermore, analyses of subcellular localization using confocal microscopy revealed that co-expression of Kir2.1 promoted the cell surface localization of Kir3.1 and Kir3.4 in HEK293T cells. In electrophysiological experiments, co-expression of Kir2.1 with Kir3.1 and/or Kir3.4 in Xenopus oocytes and HEK293T cells did not yield currents with distinguishable features. However, co-expression of a dominant-negative Kir2.1 with the wild-type Kir3.1/3.4 decreased the Kir3.1/3.4 current amplitude in Xenopus oocytes. The results indicate that Kir2.1 is capable of forming heteromultimeric channels with Kir3.1 and with Kir3.4 [966]
References
Makary SM
et al.
A difference in inward rectification and polyamine block and permeation between the Kir2.1 and Kir3.1/Kir3.4 K+ channels.
J. Physiol. (Lond.),
2005
Nov
1
, 568 (749-66).
Panama BK
et al.
Heterogeneity of IK1 in the mouse heart.
Am. J. Physiol. Heart Circ. Physiol.,
2007
Dec
, 293 (H3558-67).
Liu GX
et al.
Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes.
J. Physiol. (Lond.),
2001
Apr
1
, 532 (115-26).
Zaritsky JJ
et al.
The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes.
J. Physiol. (Lond.),
2001
Jun
15
, 533 (697-710).
Zobel C
et al.
Molecular dissection of the inward rectifier potassium current (IK1) in rabbit cardiomyocytes: evidence for heteromeric co-assembly of Kir2.1 and Kir2.2.
J. Physiol. (Lond.),
2003
Jul
15
, 550 (365-72).
Lu Z
et al.
Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel.
Nature,
1994
Sep
15
, 371 (243-6).
Stanfield PR
et al.
A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1.
J. Physiol. (Lond.),
1994
Jul
1
, 478 ( Pt 1) (1-6).
Kubo Y
et al.
Control of rectification and permeation by two distinct sites after the second transmembrane region in Kir2.1 K+ channel.
J. Physiol. (Lond.),
2001
Mar
15
, 531 (645-60).
Fujiwara Y
et al.
Ser165 in the second transmembrane region of the Kir2.1 channel determines its susceptibility to blockade by intracellular Mg2+.
J. Gen. Physiol.,
2002
Nov
, 120 (677-93).
Guo D
et al.
Interaction mechanisms between polyamines and IRK1 inward rectifier K+ channels.
J. Gen. Physiol.,
2003
Nov
, 122 (485-500).
Xie LH
et al.
Inward rectification by polyamines in mouse Kir2.1 channels: synergy between blocking components.
J. Physiol. (Lond.),
2003
Jul
1
, 550 (67-82).
Chang HK
et al.
The effects of spermine on the accessibility of residues in the M2 segment of Kir2.1 channels expressed in Xenopus oocytes.
J. Physiol. (Lond.),
2003
Nov
15
, 553 (101-12).
Yang J
et al.
Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel.
Neuron,
1995
May
, 14 (1047-54).
Dhamoon AS
et al.
Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current.
Circ. Res.,
2004
May
28
, 94 (1332-9).
Giles WR
et al.
Comparison of potassium currents in rabbit atrial and ventricular cells.
J. Physiol. (Lond.),
1988
Nov
, 405 (123-45).
Melnyk P
et al.
Differential distribution of Kir2.1 and Kir2.3 subunits in canine atrium and ventricle.
Am. J. Physiol. Heart Circ. Physiol.,
2002
Sep
, 283 (H1123-33).
Ishihara K
et al.
Heteromeric assembly of inward rectifier channel subunit Kir2.1 with Kir3.1 and with Kir3.4.
Biochem. Biophys. Res. Commun.,
2009
Mar
20
, 380 (832-7).
Xie LH
et al.
Phosphatidylinositol-4,5-bisphosphate (PIP2) regulation of strong inward rectifier Kir2.1 channels: multilevel positive cooperativity.
J. Physiol. (Lond.),
2008
Apr
1
, 586 (1833-48).
Hibino H
et al.
Inwardly rectifying potassium channels: their structure, function, and physiological roles.
Physiol. Rev.,
2010
Jan
, 90 (291-366).
Han H
et al.
Silencing of Kir2 channels by caveolin-1: cross-talk with cholesterol.
J. Physiol. (Lond.),
2014
Jul
18
, ().
Seyler C
et al.
P639Amiodarone and dronedarone inhibit inwardly rectifying Kir2.1 channels, but not Kir2.2 and Kir2.3 channels.
Cardiovasc. Res.,
2014
Jul
15
, 103 Suppl 1 (S116).
Ambrosini E
et al.
Genetically induced dysfunctions of Kir2.1 channels: implications for short QT3 syndrome and autism-epilepsy phenotype.
Hum. Mol. Genet.,
2014
May
2
, ().
Barrett-Jolley R
et al.
Direct block of native and cloned (Kir2.1) inward rectifier K+ channels by chloroethylclonidine.
Br. J. Pharmacol.,
1999
Oct
, 128 (760-6).
Romanenko VG
et al.
Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels.
Biophys. J.,
2004
Dec
, 87 (3850-61).
Kolb AR
et al.
ESCRT regulates surface expression of the Kir2.1 potassium channel.
Mol. Biol. Cell,
2014
Jan
, 25 (276-89).
Hilder TA
et al.
Conduction and block of inward rectifier K+ channels: predicted structure of a potent blocker of Kir2.1.
Biochemistry,
2013
Feb
5
, 52 (967-74).
Kulzer M
et al.
Inhibition of cardiac Kir2.1-2.3 channels by beta3 adrenoreceptor antagonist SR 59230A.
Biochem. Biophys. Res. Commun.,
2012
Jul
27
, 424 (315-20).
Ma D
et al.
Golgi export of the Kir2.1 channel is driven by a trafficking signal located within its tertiary structure.
Cell,
2011
Jun
24
, 145 (1102-15).
Contributors: Rajnish Ranjan, Nitin Khanna
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/42/ , accessed on 2025 Sep 02