Kir1.1
Description: potassium inwardly-rectifying channel, subfamily J, member 1 Gene: Kcnj1 Alias: Kir1.1, ROMK, Romk2, Kcnj1
The protein encoded by the gene KCNJ1 (also known as ROMK; ROMK1; KIR1.1) in humans is the integral membrane protein and inward-rectifier type potassium channel Kir1.1, which is member 1 of subfamily J. It is activated by internal ATP and probably plays an important role in potassium homeostasis. The encoded protein has a greater tendency to allow potassium to flow into a cell rather than out of a cell. Mutations in this gene have been associated with antenatal Bartter syndrome, which is characterized by salt wasting, hypokalemic alkalosis, hypercalciuria, and low blood pressure. Multiple transcript variants encoding different isoforms have been found for this gene. http://www.ncbi.nlm.nih.gov/gene/3758
Inwardly rectifying potassium (Kir) channels are a class of potassium channels that, at a comparable driving force, allow greater influx than efflux of potassium ions. Their high open probability at negative transmembrane voltages makes them uniquely suited to set the resting membrane potential and control cell excitability. Unlike the intrinsic gating of voltage-dependent potassium channels, inward rectification is caused by voltage-dependent block of the channel pore by cytoplasmic ions, including Mg2+ and polyamines, such as spermine and putrescine [1853]
Experimental data
Rat Kir1.1 gene in CHO host cells |
||
|
Click for details 
25 °Cshow 46 cells |
Click for details 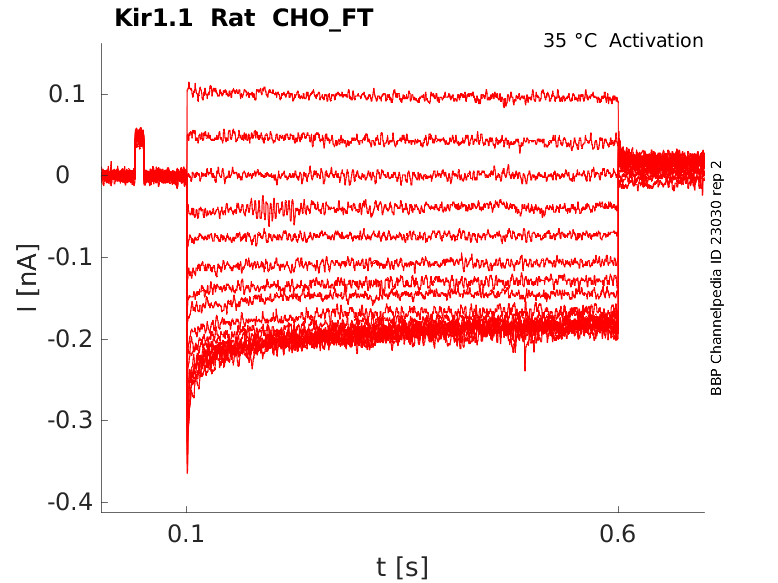
35 °Cshow 10 cells |
|
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_000220.6 | 2333 | |
| Mouse | NM_001168354.1 | 3075 | |
| Rat | NM_017023.2 | 2999 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Structural model of an inward rectifier potassium channel

GL: Gating loop; HBC: Helix bundle crossing; RC: Rectification controller; RM: Rectification modulator; SF: Selectivity filter; TM1 and 2: Transmembrane domains 1 and 2 The inward rectifier Kir1.1 (ROMK) family is gated by both internal pH and external K, where the putative pH gate is formed by the convergence of leucine side chains, near the inner helical bundle crossing at L160-Kir1.1. Structural disruption of the Kir1.1 bundle-crossing pH gate prevents both inactivation by low external K and reactivation by high external K. [178]
Kir1.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Single Channel Conductance
Patch-clamp experiments demonstrated that two types of K channels, a 30- to 40- and a 70- to 80-pS, are expressed in the apical membrane of the TAL (thick ascending limb ). It is well established that the 30-pS K channel is related to ROMK because it has similar biophysical properties and regulatory mechanisms to that in native tubules. However, it is not clear whether ROMK is also involved in forming the apical 70-pS K channel. [1843]
Single Channel Conductance of Kir1.1 in Apical Membrane of Mouse Cortical TAL

Rat ROMK1 Expressed in HEK293 Cells

Kinetics
The kinetics of the Kir family do not follow the Hodgkin Huxley Kinetics model. This is because its kinetics depends not only on the membrane potential but also on the equilibrium potential for K+. The channel conductance increases as the concentration of extra cellular K+ increases [1844]
Kir1 Kinetics in A gambiae Mosquitos
when bathed in a control (ND96) solution, heterologous expression of AgKir1 channels in Xenopus oocytes gave rise to functional channels exhibiting spontaneous large inward and small outward currents between −140 mV and −80 mV. Moreover, the spontaneous resting membrane potential of the AgKir1-expressing oocytes (−96.9 ± 1.3 mV) was (1) hyperpolarized compared to the H2O-injected oocytes (−42.0 ± 2.5 mV), and (2) close to the estimated Nernst potential for K+ (−102 mV). The inward AgKir1 channel currents increase with increased extracellular K+ concentrations and are inhibited by barium. These functional characteristics are canonical of most animal Kir channels, and very similar to those of AeKir1. Thus, AgKir1 functions as an inward rectifier K+ channel [1852]
Human Kir1.1 Expressed in CHO Cells
Human Kir1.1, stably expressed in CHO cells, produced large, stable currents on the IonWorks HT platform. Using a 0.1-s ramp from −100 to +100 mV as the voltage command, the recorded currents displayed inward rectifying properties and were inhibited by the addition of 5 mM BaCl2. Incubation with 5 mM BaCl2 is expected to completely block currents conducted by Kir1.1 channels, and the remaining current is presumed to be a nonspecific “leak” current, resulting from the low resistance seal between the cell and the plastic substrate used to manufacture the disposable PatchPlate™. To minimize the contribution of leak currents to currents recorded from hKir1.1-expressing cells, the preferred protocol (protocol 2) combined near-physiological extracellular potassium concentrations (10 mM) with a test pulse voltage of 0 mV [1853]
Hodgkin and Huxley Model Prediction for Kir1.1

Kir1.1 Expressed in Rat Brain
The Kir1.1 channel has been seen to be expressed in several parts of the adult rat CNS. This includes the Olfactory Bulb, Granular Layer and Piriform Cortex [983]
Kir1 Predominantly Expressed in Kidney
Kir1.1, the product of the KCNJ1 gene, also known as renal outer medullary potassium (ROMK) channel, is unique in that its expression appears to be highly restricted to the kidney. Specifically, Kir1.1 channels are located at the apical membrane of epithelial cells lining the thick ascending loop of Henle (TALH) and the cortical collecting duct (CCD)13 and represent the molecular correlate of the small-conductance secretory potassium channel [1853]
Kir1.1 Expressed in Distal Convoluted Tubule
ROMK expression along the entire distal nephron, localizing to distal convoluted tubule regions, DCT1 and DCT2; the connecting tubule (CNT); and cortical collecting duct (CD). ROMK is diffusely distributed in intracellular compartments and at the apical membrane of each tubular region. Apical labeling was significantly increased by high-K diet in DCT2, CNT1, CNT2, and CD (P < 0.05) but not in DCT1. High-K diet causes a large increase in apical expression of ROMK in DCT2, CNT, and CD but not in DCT1, indicating that different regulatory mechanisms are involved in K diet-regulated ROMK channel functions in the distal nephron. Wade [908]
SubCellular Distribution of Rat Kir1.1 (ROMK1) in Neuron

Potassium Recycling
The ROMK channel plays an important role in K recycling in the thick ascending limb (TAL) and K secretion in the connecting tubule (CT) and cortical collecting duct (CCD). It is generally agreed that ROMK channels are mainly responsible for K secretion under normal conditions. [1843]
At the TALH (thick ascending loop of Henle), Kir1.1 participates in potassium recycling across the luminal membrane that is critical for the function of the furosemide-sensitive Na+/K+/2Cl− co-transporter, the rate-determining step for salt reuptake in this part of the nephron. At the CCD, Kir1.1 provides a pathway for potassium secretion that is tightly coupled to sodium uptake through the amiloride-sensitive epithelial sodium channel. Several dozen mutations in the KCNJ1 gene that cause loss of channel function have been identified in individuals with Bartter's syndrome type II, an autosomal recessive life-threatening disorder [1853]
PIP Metabolism
Kir channels are only active when associated with phosphatidylinositol 4,5-bisphosphate (PIP) and therefore their activity is intimately coupled to the complex cellular metabolism of PIPs. Baukrowitz [904], Kobrinski [905], Xie [906]. The physiological importance of this phosphoinositide regulation is underlined by the observation that mutations in Kir1.1 channel which impair PIP2 interactions can lead to disease states such as the Bartter’s syndrome. Lopes [907]
Disease and Pharmacology
Inward rectifier potassium (Kir) channels have been postulated as therapeutic targets for several common disorders including hypertension, cardiac arrhythmias and pain. With few exceptions, however, the small-molecule pharmacology of this family is limited to nonselective cardiovascular and neurologic drugs with off-target activity toward inward rectifiers. Consequently, the actual therapeutic potential and 'drugability' of most Kir channels has not yet been determined experimentally. The purpose of this review is to provide a comprehensive summary of publicly disclosed Kir channel small-molecule modulators and highlight recent targeted drug-discovery efforts toward Kir1.1 and Kir2.1 [1846]
Hypertension
Human and rodent genetic studies provide a large body of evidence which suggests that inhibitors of the kidney potassium channel, ROMK, will represent novel diuretics for the treatment of hypertension. The search for potent and selective ROMK inhibitors has recently yielded compounds that display efficacy in animal models, providing the first pharmacological validation of ROMK as a novel diuretic target.
Co expression with Kir7.1
In a high-throughput screen for small-molecule modulators of ROMK, we previously identified a potent and moderately selective ROMK antagonist, 7,13-bis(4-nitrobenzyl)-1,4,10-trioxa-7,13-diazacyclopentadecane (VU590), that also inhibits Kir7.1. Because ROMK and Kir7.1 are coexpressed in the nephron, VU590 is not a good probe of ROMK function in the kidney. Here we describe the development of the structurally related inhibitor 2,2'-oxybis(methylene)bis(5-nitro-1H-benzo[d]imidazole) (VU591), which is as potent as VU590 but is selective for ROMK over Kir7.1 and more than 65 other potential off-targets [1860]
Ph
The inward rectifier Kir1.1 (ROMK) family is gated by both internal pH and external K. Sackin [178]
Calcium and Magnesium
Kir1.1 inactivation, associated with transient internal acidification, is strongly dependent on external K, Ca, and Mg. Here, we show that in 1 mM K, a 15 min internal acidification (pH 6.3) followed by a 30 min recovery (pH 8.0) produced 84 ± 3% inactivation in 2 mM Ca but only 18 ± 4% inactivation in the absence of external Ca and Mg. In 100 mM external K, the same acidification protocol produced 29 ± 4% inactivation in 10 mM external Ca but no inactivation when extracellular Ca was reduced below 2 mM (with 0 Mg) [1858]
Ifenprodil
Kir1.1 and Kir2.1 channels are insensitive to ifenprodil [179]
E132, F127, and R128
Three residues (E132, F127, and R128) at the outer mouth of Kir1.1b directly affected inward rectifier gating by external K, independent of pH gating. Each of the individual mutations E132Q, F127V, F127D, and R128Y changed the normal K dependence of macroscopic conductance from hyperbolic (Km = 6 ± 2 mM) to linear, up to 500 mM, without changing the hyperbolic K dependence of single-channel conductance. This suggests that E132, F127, and R128 are responsible for maximal Kir1.1b activation by external K. In addition, these same residues were also essential for recovery of Kir1.1b activity after complete removal of external K by 18-Crown-6 polyether [1857]
Phosphatidylinositol 3,4-bisphosphate
Phosphatidylinositol 3,4-bisphosphate has an inhibitory effect on Kir1.1. [180]
Kir channels are only active when associated with phosphatidylinositol 4,5-bisphosphate (PIP) and therefore their activity is intimately coupled to the complex cellular metabolism of PIPs. Baukrowitz [904], Kobrinski [905], Xie [906]. The physiological importance of this phosphoinositide regulation is underlined by the observation that mutations in Kir1.1 channel which impair PIP2 interactions can lead to disease states such as the Bartter’s syndrome. Lopes [907]
Oleoyl-CoA,
ong chain fatty acid esters of coenzyme A (LC-CoA), e.g. oleoyl-CoA, potently and reversibly inhibits Kir1.1. [180]
K+
Also, it has been shown that changes in extracellular K concentrations can affect the sensitivity of ROMK channels to cell pH [1843]
Kir1 Inhibitors
The only inhibitors of Kir1.1 disclosed to date are variants of the bee venom peptide tertiapin (TPN) and two small molecules, VU590 and VU591, identified in a screening campaign at Vanderbilt University [1853]
References
Sackin H
et al.
External K activation of Kir1.1 depends on the pH gate.
Biophys. J.,
2007
Jul
15
, 93 (L14-6).
Kobayashi T
et al.
Inhibition of G protein-activated inwardly rectifying K+ channels by ifenprodil.
Neuropsychopharmacology,
2006
Mar
, 31 (516-24).
Rapedius M
et al.
Long chain CoA esters as competitive antagonists of phosphatidylinositol 4,5-bisphosphate activation in Kir channels.
J. Biol. Chem.,
2005
Sep
2
, 280 (30760-7).
Jin W
et al.
Mechanisms of inward-rectifier K+ channel inhibition by tertiapin-Q.
Biochemistry,
1999
Oct
26
, 38 (14294-301).
Baukrowitz T
et al.
K(ATP) channels: linker between phospholipid metabolism and excitability.
Biochem. Pharmacol.,
2000
Sep
15
, 60 (735-40).
Kobrinsky E
et al.
Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+-current desensitization.
Nat. Cell Biol.,
2000
Aug
, 2 (507-14).
Xie LH
et al.
Phospholipase C-linked receptors regulate the ATP-sensitive potassium channel by means of phosphatidylinositol 4,5-bisphosphate metabolism.
Proc. Natl. Acad. Sci. U.S.A.,
1999
Dec
21
, 96 (15292-7).
Lopes CM
et al.
Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies.
Neuron,
2002
Jun
13
, 34 (933-44).
Wade JB
et al.
Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium.
Am. J. Physiol. Renal Physiol.,
2011
Jun
, 300 (F1385-93).
Karschin C
et al.
IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain.
J. Neurosci.,
1996
Jun
1
, 16 (3559-70).
Jiang C
et al.
An alternative approach to the identification of respiratory central chemoreceptors in the brainstem.
,
2001
Dec
, 129 (141-57).
Shuck ME
et al.
Cloning and characterization of two K+ inward rectifier (Kir) 1.1 potassium channel homologs from human kidney (Kir1.2 and Kir1.3).
J. Biol. Chem.,
1997
Jan
3
, 272 (586-93).
Wang WH
Regulation of ROMK (Kir1.1) channels: new mechanisms and aspects.
Am. J. Physiol. Renal Physiol.,
2006
Jan
, 290 (F14-9).
Isomoto S
et al.
Inwardly rectifying potassium channels: their molecular heterogeneity and function.
Jpn. J. Physiol.,
1997
Feb
, 47 (11-39).
Schulte U
et al.
Gating of inward-rectifier K+ channels by intracellular pH.
Eur. J. Biochem.,
2000
Oct
, 267 (5837-41).
Bhave G
et al.
Small-molecule modulators of inward rectifier K+ channels: recent advances and future possibilities.
Future Med Chem,
2010
May
, 2 (757-74).
Garcia ML
et al.
Targeting the inward-rectifier potassium channel ROMK in cardiovascular disease.
Curr Opin Pharmacol,
2014
Apr
, 15 (1-6).
Raphemot R
et al.
Molecular and functional characterization of Anopheles gambiae inward rectifier potassium (Kir1) channels: A novel role in egg production.
Insect Biochem. Mol. Biol.,
2014
May
20
, 51C (10-19).
Felix JP
et al.
The Inwardly Rectifying Potassium Channel Kir1.1: Development of Functional Assays to Identify and Characterize Channel Inhibitors.
Assay Drug Dev Technol,
2012
Aug
10
, ().
Lu M
et al.
CFTR is required for PKA-regulated ATP sensitivity of Kir1.1 potassium channels in mouse kidney.
J. Clin. Invest.,
2006
Mar
, 116 (797-807).
Nadeau H
et al.
ROMK1 (Kir1.1) causes apoptosis and chronic silencing of hippocampal neurons.
J. Neurophysiol.,
2000
Aug
, 84 (1062-75).
Sackin H
et al.
Residues at the outer mouth of Kir1.1 determine K-dependent gating.
Biophys. J.,
2012
Jun
20
, 102 (2742-50).
Sackin H
et al.
Modulation of Kir1.1 inactivation by extracellular Ca and Mg.
Biophys. J.,
2011
Mar
2
, 100 (1207-15).
Abbas L
et al.
Functional and developmental expression of a zebrafish Kir1.1 (ROMK) potassium channel homologue Kcnj1.
J. Physiol. (Lond.),
2011
Mar
15
, 589 (1489-503).
Bhave G
et al.
Development of a selective small-molecule inhibitor of Kir1.1, the renal outer medullary potassium channel.
Mol. Pharmacol.,
2011
Jan
, 79 (42-50).
Hibino H
et al.
Inwardly rectifying potassium channels: their structure, function, and physiological roles.
Physiol. Rev.,
2010
Jan
, 90 (291-366).
Credits
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/41/ , accessed on 2026 Feb 10