Kv8.1
Description: potassium channel, subfamily V, member 1 Gene: Kcnv1 Alias: Kv8.1, kcnv1, HNKA
Kv8.1 (also known as KCNV1; HNKA; KCNB3; KV2.3.), encoded by the gene KCNV1, is a member of the potassium voltage-gated channel subfamily V. Kv8.1 is essentially present in the brain and its role might be to inhibit the function of a particular class of outward rectifier potassium channel types. NCBI
Experimental data
Rat Kv8.1 gene in CHO host cells datasheet |
||
|
Click for details 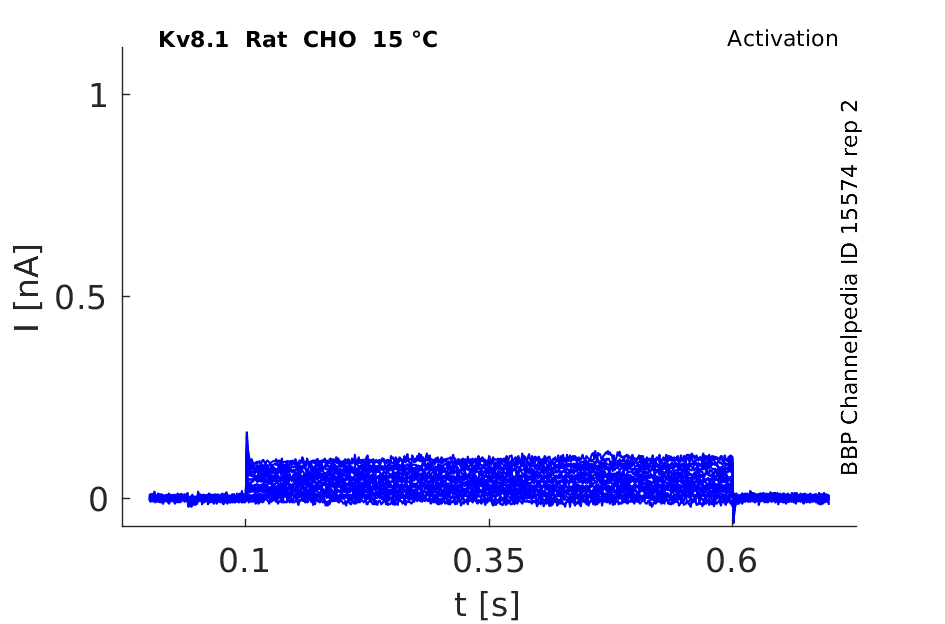
15 °Cshow 65 cells |
Click for details 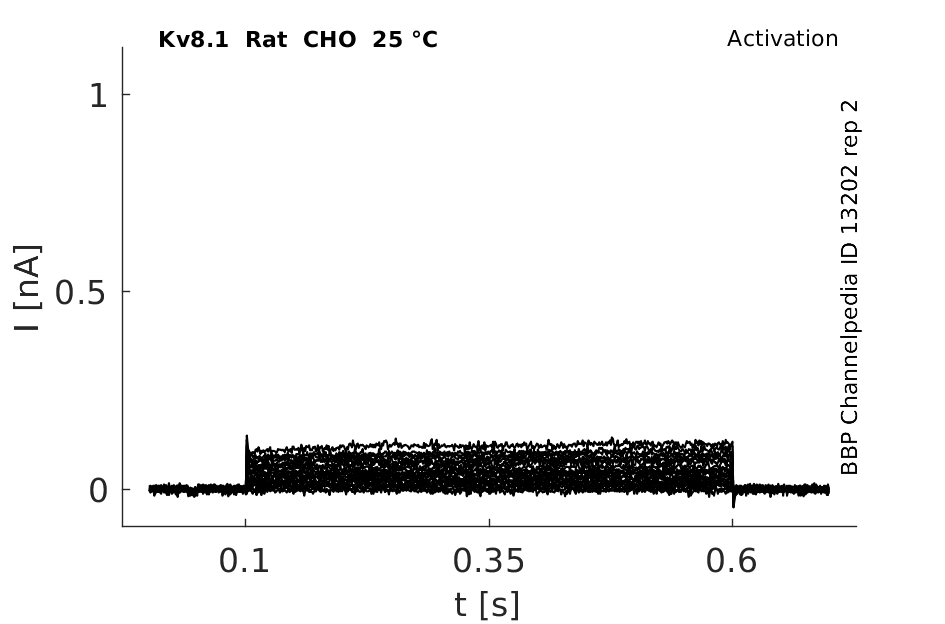
25 °Cshow 72 cells |
Click for details 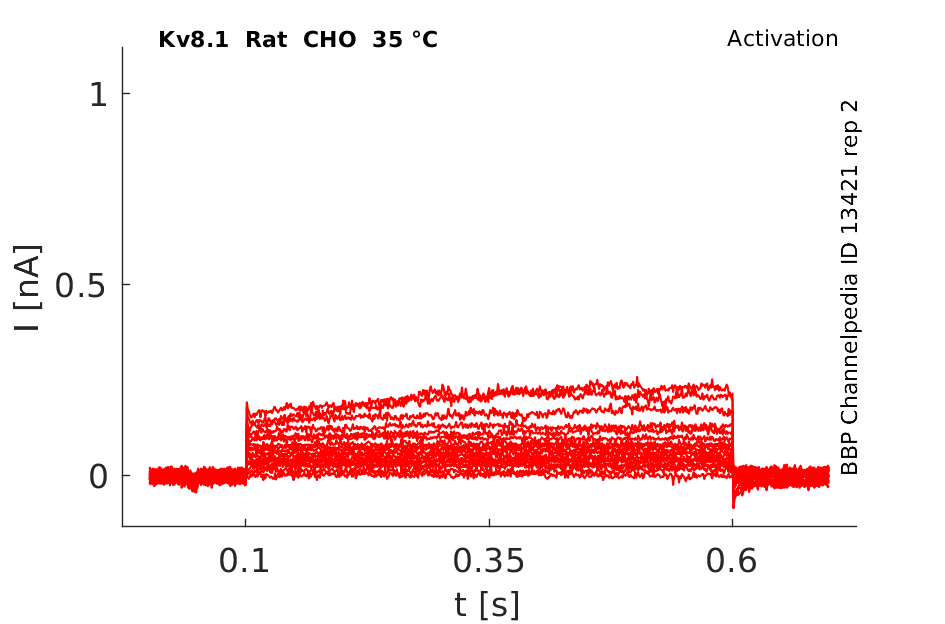
35 °Cshow 82 cells |
The whole genomic structure of KCNV1, and 5V exon and promoter sequence can be found in [727]. Analysis of the KCNV1 promoter revealed the essential elements for transcription. An alternative 3Vend of the transcript with preceding poly(A) addition signal was also detected. [727]
KCNV1 was previously reported to comprise three exons, but the present study demonstrated the existence of both a novel 5′ exon and the real promoter sequence in the upstream region of that exon [727]
KCNV1 is located on chromosome 8q23.3, the susceptibility region for benign adult familial myoclonic epilepsy (BAFME) [728], making this gene an interesting candidate for the disease.
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_014379.4 | 6912 | |
| Mouse | NM_026200.3 | 4496 | |
| Rat | NM_021697.2 | 3956 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Kv8.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Visual Representation of Kv8.1 Structure
Methodology for visual representation of structure available here
Expression of Kv8. 1 subunit in Xenopus laevis oocytes
Injections of Xenopus oocytes with the Kv8.1 cRNA were usedtotestiftheKv8.1 protein is able to give rise to K+ currents. Following test pulses from -130 to +60 V, neither outward nor inward currents could be detected. Modification of the external pH as well as of external ionic concentrations, addition of oxidative (H202) or reducing agents (dithiothreitol) and activation of different protein kinases (kinase A, kinase C) were assayed to reveal an expression of K+ channels, but none of these treatments led to current detection.Also,attempts to detect K+currents from CHO and COS-7cells after transfection with a Kv8.1-expressing vector were unsuccessful [726]
Silent Current Kv8.1 interacts with Kv2.1 and Kv2.2

Kv8.1 does not influence Kv3.4 kinetics

Expression of Kv8.1 in Brain
Kv8.1 is essentially present in the brain where it is located mainly in layers I,IV and VI of the cerebral cortex, in hippocampus,in CA1-CA4 pyramidal cell layer as well in granule cells of the dentate gyrus, in the granule cell layer and in the Purkinje cell layer of the cerebellum [726]
Kv8.1 subunit is normally retained in cytoplasmic compartments. It requires coexpression with Kv2.2 to bring the subunit to the plasma membrane. [176]
The S6 segment of Kv8.1 is atypical and contains the structural elements that modify inactivation of Kv2 channels. [167]
Kv8.1 induces no inhibition of the Kv2.2 current in COS mammalian cell line but instead produces a drastic modification of the kinetic properties of Kv2.2 and particularly of its inactivation. The same effect can be seen on Kv2.1 and Kv2.2 currents in Xenopus oocytes expressing moderate levels of Kv8.1, while a total inhibition is seen with higher levels of Kv8.1. Therefore, depending on its level of expression, Kv8.1 can either modify the kinetics of the Kv2 channels or completely abolish their activity. Site-directed mutagenesis has been used to show that Kv8.1 effects on the Kv2 current are mediated by the presence of singular amino acids located in the S6 domain of the Kv8.1 subunit. [176]
Kv1, Kv2, Kv3 and Kv4 ion channel
The Kv8.1 (KCNV1) protein displays approximately 40% sequence identity with other K+ channel subunits. However, the KCNV1 subunit does not generate K+ channel activity in Xenopus oocytes, instead acting to suppress Kv2 and Kv3 channels. In COS cells, KCNV1 does not inhibit the Kv2.2 current when cotransfected with Kv2.2, but changes the kinetic properties of Kv2.2 [727]

Abolishes Kv2.1 Kv2.2 and Kv3.4 Channels
At lower levels, Kv8.1 associates with Kv2.1 and Kv2.2 to form hybrid Kv8.1/Kv2 channels, which have new biophysical properties and more particularly modified properties of the inactivation process as compared with homopolymers of Kv2.1 or Kv2.2 channels. The same effects have been seen by coexpressing the Kv8.1 subunit and the Kv2.2 subunit in COSm6 cells. In these cells, Kv8.1 expressed alone remains in intracellular compartments, but it can reach the plasma membrane when it associates with Kv2.2, and it then also forms new types of Kv8.1/Kv2. 2 channels. Present results indicate that Kv8.1 when expressed at low concentrations acts as a modifier of Kv2.1 and Kv2.2 activity, while when expressed at high concentrations in oocytes it completely abolishes Kv2.1, Kv2.2, or Kv3.4 K+ channel activity [176]
Mn2+
We have also shown that a random mutagenesis approach based on PCR in Mn2+-containing buffer is useful for introducing mutations and determining important sequence elements in promoter analysis [727]
References
Activation of Slo1 BK channels by Mg2+ coordinated between the voltage sensor and RCK1 domains.
Nat. Struct. Mol. Biol., 2008 Nov , 15 (1152-9).
Salinas M
et al.
Modes of regulation of shab K+ channel activity by the Kv8.1 subunit.
J. Biol. Chem.,
1997
Mar
28
, 272 (8774-80).
Salkoff L
et al.
An essential 'set' of K+ channels conserved in flies, mice and humans.
Trends Neurosci.,
1992
May
, 15 (161-6).
Hugnot JP
et al.
Kv8.1, a new neuronal potassium channel subunit with specific inhibitory properties towards Shab and Shaw channels.
EMBO J.,
1996
Jul
1
, 15 (3322-31).
Ebihara M
et al.
Structural characterization and promoter analysis of human potassium channel Kv8.1 (KCNV1) gene.
Gene,
2004
Jan
21
, 325 (89-96).
Mikami M
et al.
Localization of a gene for benign adult familial myoclonic epilepsy to chromosome 8q23.3-q24.1.
Am. J. Hum. Genet.,
1999
Sep
, 65 (745-51).
Sano A
et al.
Positional candidate approach for the gene responsible for benign adult familial myoclonic epilepsy.
Epilepsia,
2002
, 43 Suppl 9 (26-31).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/28/ , accessed on 2024 May 08