Kir3.2
Description: potassium inwardly-rectifying channel, subfamily J, member 6 Gene: Kcnj6 Alias: Kir3.2, BIR1, GIRK2, KATP2, KCNJ7, Kcnj6
In mammals, G-protein-coupled inwardly rectifying potassium channels - such as Kir 3.2 - are composed of four subunits that are individual members of the Kir3 (formerly GIRK) family and form homomeric or heteromeric complexes (Liao et al., 1996 [981]; Wischmeyer et al., 1997 [982]).
KCNJ6 (also known as BIR1; GIRK2; KATP2; KCNJ7; GIRK-2; KATP-2; KIR3.2; hiGIRK2; MGC126596) encodes Kir3.2, an integral membrane protein, inward-rectifier type potassium channel, subfamily J, member 6. The encoded protein, which has a greater tendency to allow potassium to flow into a cell rather than out of a cell, is controlled by G-proteins and may be involved in the regulation of insulin secretion by glucose. It associates with two other G-protein-activated potassium channels to form a heteromultimeric pore-forming complex.
http://www.ncbi.nlm.nih.gov/gene/3763
Experimental data
Rat Kir3.2 gene in CHO host cells |
||
|
Click for details 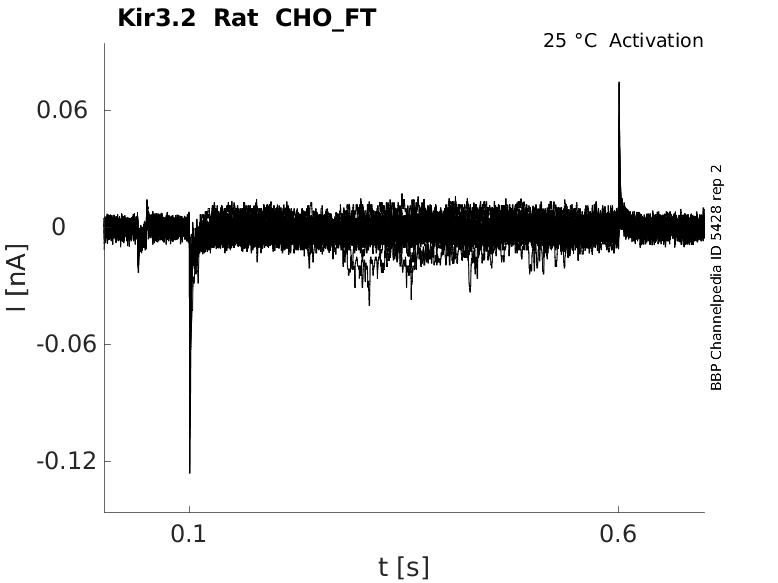
25 °Cshow 55 cells |
Click for details 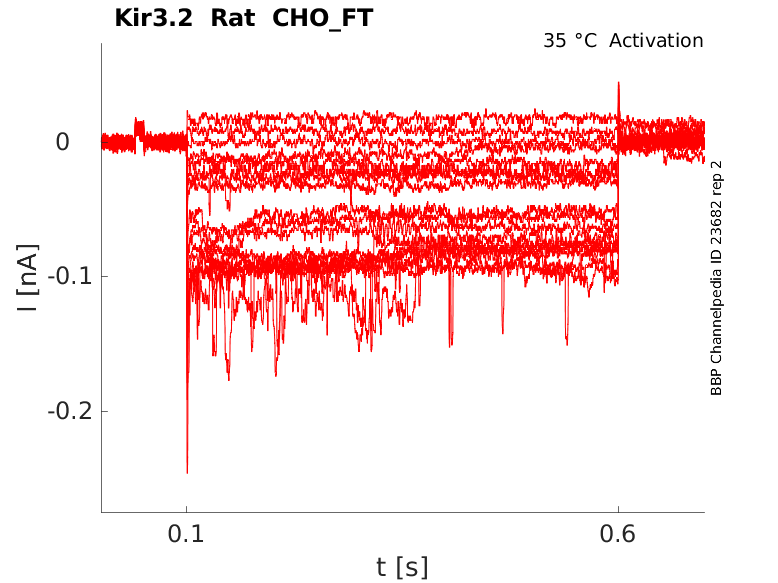
35 °Cshow 26 cells |
|
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_002240.5 | 19659 | |
| Mouse | NM_010606.2 | 3086 | |
| Rat | NM_013192.2 | 16380 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Structure
Kir3.2 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Kir3.2 is the predominant Kir3 subunit in the midbrain (Karschin et al., 1996 [983]; Chen et al., 1997 [984]; Murer et al., 1997 [985]; Schein et al., 1998 [986]).
Kir3.2 ( = GIRK2) is important for opioid receptor transmission. Loetsch [973]
The importance of the strong expression of Kir3.2 in midbrain neurons is documented by the fact that a point mutation in the pore of the mouse Kir3.2 channel, leading to a loss of ion selectivity, results in the weaver (wv) phenotype. Such mice suffer from aberrant postnatal development and death of several classes of neurons including the dopaminergic neurons of the SNc (Liao et al., 1996 [981]; Surmeier et al., 1996 [987]).
The heterogeneously distributed Kir3.2 channel proteins could help to discriminate the dopaminergic neurons of ventral tegmental area and substantia nigra pars compacta. Eulitz [973]
G-Protein
The activation of GIRK channels is mediated by a pertussis toxin-sensitive G protein, via a direct membrane-delimited pathway without involving intracellular second messenger systems. The G-beta-gamma subunit plays an important physiological role in the activation by direct binding to multiple regions of GIRK channels (Huang [979]). Signaling via the G-protein-mediated pathway is regulated by a recently identified gene family, known to encode RGS proteins (regulators of G-protein signaling) (Druey [980]).
RGS4 reduces the basal GIRK1/GIRK2 current and strongly increases the percentage agonist-evoked K+ conductance. RGS4 reconstitutes the native gating kinetics by accelerating GIRK1/GIRK2 channel deactivation. Ulens [194]
GIRK2 but not GIRK3 can be activated by G protein subunits Gj3, and G-y2 in Xenopus oocytes. Kofuji [195]
References
Ulens C
et al.
Changes in GIRK1/GIRK2 deactivation kinetics and basal activity in the presence and absence of RGS4.
Life Sci.,
2000
Sep
29
, 67 (2305-17).
Kofuji P
et al.
Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by G beta gamma subunits and function as heteromultimers.
Proc. Natl. Acad. Sci. U.S.A.,
1995
Jul
3
, 92 (6542-6).
Styer AM
et al.
G protein {beta}{gamma} gating confers volatile anesthetic inhibition to Kir3 channels.
J. Biol. Chem.,
2010
Dec
31
, 285 (41290-9).
Lötsch J
et al.
A KCNJ6 (Kir3.2, GIRK2) gene polymorphism modulates opioid effects on analgesia and addiction but not on pupil size.
Pharmacogenet. Genomics,
2010
May
, 20 (291-7).
Eulitz D
et al.
Heterogeneous distribution of kir3 potassium channel proteins within dopaminergic neurons in the mesencephalon of the rat brain.
Cell. Mol. Neurobiol.,
2007
May
, 27 (285-302).
Kulik A
et al.
Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells.
J. Neurosci.,
2006
Apr
19
, 26 (4289-97).
Mathie A
et al.
What are the roles of the many different types of potassium channel expressed in cerebellar granule cells?
Cerebellum,
2003
, 2 (11-25).
Gerstin KM
et al.
Mutation of KCNK5 or Kir3.2 potassium channels in mice does not change minimum alveolar anesthetic concentration.
Anesth. Analg.,
2003
May
, 96 (1345-9, table of contents).
Torrecilla M
et al.
G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons.
J. Neurosci.,
2002
Jun
1
, 22 (4328-34).
Bradley KK
et al.
Kir3.1/3.2 encodes an I(KACh)-like current in gastrointestinal myocytes.
Am. J. Physiol. Gastrointest. Liver Physiol.,
2000
Feb
, 278 (G289-96).
Huang CL
et al.
Binding of the G protein betagamma subunit to multiple regions of G protein-gated inward-rectifying K+ channels.
FEBS Lett.,
1997
Apr
1
, 405 (291-8).
Druey KM
et al.
Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family.
Nature,
1996
Feb
22
, 379 (742-6).
Liao YJ
et al.
Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain.
J. Neurosci.,
1996
Nov
15
, 16 (7137-50).
Wischmeyer E
et al.
Subunit interactions in the assembly of neuronal Kir3.0 inwardly rectifying K+ channels.
Mol. Cell. Neurosci.,
1997
, 9 (194-206).
Karschin C
et al.
IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain.
J. Neurosci.,
1996
Jun
1
, 16 (3559-70).
Chen SC
et al.
Developmental expression of the GIRK family of inward rectifying potassium channels: implications for abnormalities in the weaver mutant mouse.
Brain Res.,
1997
Dec
19
, 778 (251-64).
Murer G
et al.
An immunocytochemical study on the distribution of two G-protein-gated inward rectifier potassium channels (GIRK2 and GIRK4) in the adult rat brain.
Neuroscience,
1997
Sep
, 80 (345-57).
Schein JC
et al.
Girk2 expression in the ventral midbrain, cerebellum, and olfactory bulb and its relationship to the murine mutation weaver.
Dev. Biol.,
1998
Dec
15
, 204 (432-50).
Surmeier DJ
et al.
The weaver mutation of GIRK2 results in a loss of inwardly rectifying K+ current in cerebellar granule cells.
Proc. Natl. Acad. Sci. U.S.A.,
1996
Oct
1
, 93 (11191-5).
Credits
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/47/ , accessed on 2024 Apr 27