Kv2.2
Description: potassium voltage gated channel, Shab-related subfamily, member 2 Gene: Kcnb2 Alias: Kv2.2, kcnb2, kcnb2-A
Kv2.2, encoded by the gene KCNB2, is a member of the potassium voltage-gated channel subfamily B. Kv2.2 is a major constituents of the somatic delayed rectifier potassium current
Experimental data
Rat Kv2.2 gene in CHO host cells datasheet |
||
|
Click for details 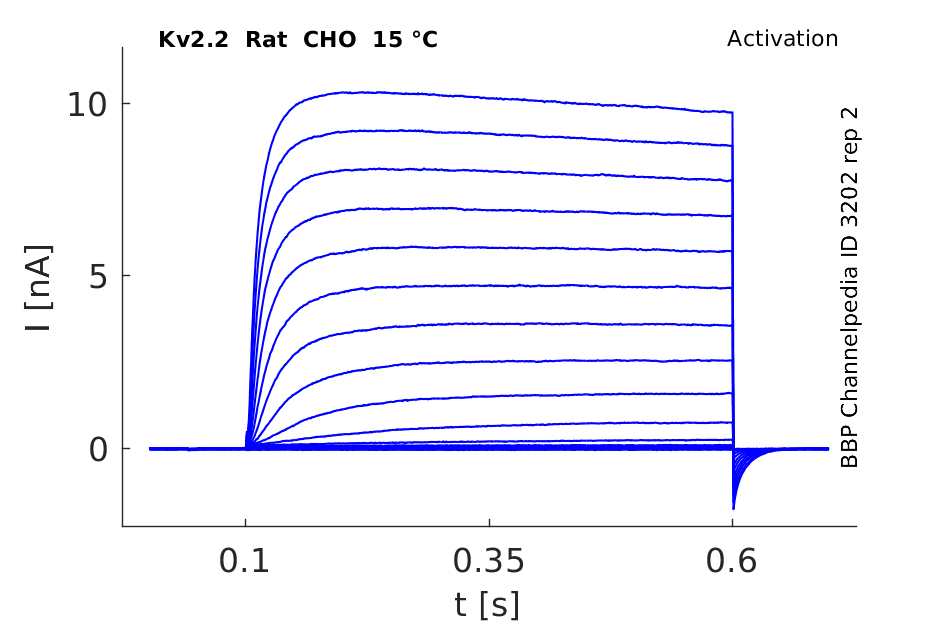
15 °Cshow 27 cells |
Click for details 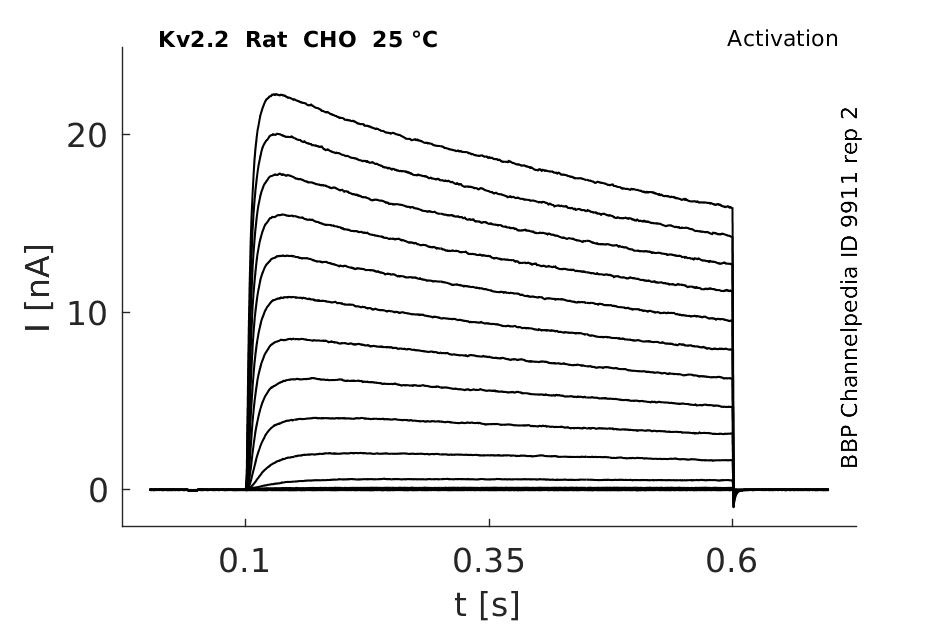
25 °Cshow 93 cells |
Click for details 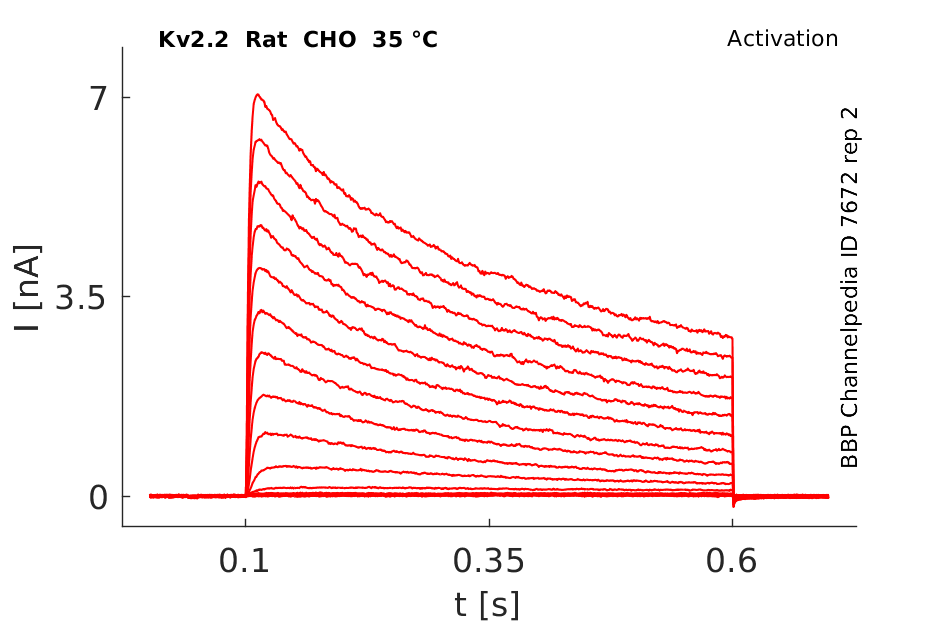
35 °Cshow 84 cells |
A variety of rat Kv2.2 has been found, called Kv2.2-long.[393]
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_004770.3 | 3748 | |
| Mouse | NM_001098528.3 | 15494 | |
| Rat | NM_054000.2 | 3230 |
Protein Isoforms
Isoforms
Post-Translational Modifications
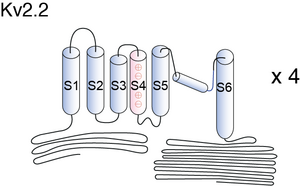
Visual Representation of Kv2.2 Structure
Methodology for visual representation of structure available here
Kv2.2 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
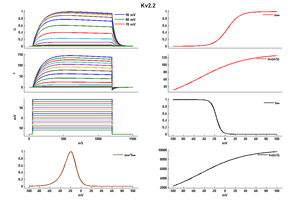
KV2.2 KINETICS
So the slow activation of Kv2 channels means that Kv2 conductance is unlikely to contribute to single APs when AP half-widths are around 0.5 ms [309] Kv2.2 mRNA injected into Xenopus oocytes resulted in the expression of a slowly activating K+ current mediated by 15 symmetrical K+ single channels. [278]
Kv2.2-containing channels are crucial for maintaining AP amplitude by regulating the interspike potential during high frequency firing in medial nucleus of the trapezoid body neurons. [309]
an in situ study revealed that elimination of Kv2 channel resulted in a reduction of the density of noninactivating potassium current and a prolonged impulse duration. In contrast, suppression of noninactivating current carried by Kv1 channels was much less effective in increasing action potential durations [1625]
Effect of Citalopram on Kv2.2 Kinetics

Single Channel Conductance
Kv 2.2 has a single channel conductance of 15ps [278]
Pathway of Kv2.2 inhibition by resveratrol in HEK cells
The modes of inhibition of Kv2 channels by the phytoalexin resveratrol (REV) was studied using the patch-clamp technique on cultured cerebellar granule cells and HEK293 cells transfected with the genes coding for Kv2.1 or Kv2.2 respectively. The results showed that REV suppressed Kv2.2 but not Kv2.1 currents. Inhibition of Kv2.2 was fast, reversible and slightely concentration-dependent with shifted activation and inactivation curves. Inhibition of the GPR30 receptor diminished the effect of REV on Kv2.2 channels indicating that REV regulates Kv2.2 through the estrogen receptor GPR30-mediated PKC pathway.[1656]
Kv2.2 expressed in HEK293 cells
Recordings on Kv2.2, heterologously expressed HEK293 cells, reveal that Kv2.2 is largely refractory to stimuli that in Kv2.1 will trigger robust changes in clustering and function.[2061]
HODGKIN & HUXLEY MODEL KV2.2

Model Kv2.2 (ID=24)
| Animal | Xenopus | |
| CellType | oocyte | |
| Age | 0 Days | |
| Temperature | 23.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [278] S D Koh et. al; Am. J. Physiol. 1998 May | |
| mpower | 1.0 | |
| m Inf | 1/(1+exp(((v -(5.000))/(-12.000)))) | |
| m Tau | 130.000/(1+exp(((v -(-46.560))/(-44.140)))) | |
| hpower | 1.0 | |
| h Inf | 1/(1+exp(((v -(-16.300))/(4.800)))) | |
| h Tau | 10000.000/(1+exp(((v -(-46.560))/(-44.140)))) | |
Kv2.2 Expression in Brain
Previous studies reported that Kv2.2 is detected in the cerebral cortex, cerebellum, hippocampus, striatum, brain stem, and thalamus [309]. In this article we report a novel and abundant expression of Kv2.2 in the basal forebrain of the rat and mouse [1736]
Kv2.2 Expression in Tissue
Kv2.2 channels were found in human and rat alpha and delta but not beta pancreatic islet cells. [6]
Kv2.2 is expressed in smooth muscle cells in all regions of the canine gastrointestinal tract and in several vascular tissues. [278]
Kv2.2 channels were found in medial nucleus of the trapezoid body neurons [309] and cortical pyramidal neurons [393].
Kv2.2 protein in developing Xenopus laevis were localizes in post-mitotic cells at early stages of differentiation in ventrolateral regions of the brain and spinal cord, especially in areas associated with growing axonal tracts. Moreover, Kv2.2 protein was found in the retina, inner ear, and olfactory epithelium shortly after these sensory tissues began to form. Many neurons and sensory cells begin to synthesize Kv2.2 protein at early stages of post-mitotic differentiation and maintain expression thereafter. [394]
A large subpopulation of neurons within the magnocellular preoptic area (MCPO) and the horizontal limb of the diagonal band of Broca (HDB) of the basal forebrain express the Kv2.2 voltage-gated potassium channel [1623]
Kv2 in S1 Ctx pyramidal neurons###
Kv2.1 and Kv2.2 expressed in distinct cortical layers and pyramidal cell types associated with corticostriatal pathways.While Kv2.1 is expressed in all cortical layers, Kv2.2 is predominantly expressed in L2andL5a and absent from L4 of the primary somatosensory cortex. Circuit-specificity of Kv2.1 and Kv2.2 is indicated by dinstinct expression patterns in intratelencephalic and pyramidal tract neurons of L5 as was demostrated by immunostaining and voltage-clamp recording[2061]
Kv2.2 Distribution in Neuron
Kv2.2 is highly expressed in axon initial segments of medial nucleus of the trapezoid body neurons. [309]
Kv2.1 and Kv2.2-long were both found in the somata and proximal dendrites of cortical pyramidal neurons from rat. [393]
In neurons of developing Xenopus laevis, Kv2.2 is present in the axon, in contrast to the dendritic localization of Kv2.1. [394]
NEURONAL FUNCTION
Kv2.1 and Kv2.2-long in rat cortical pyramidal neurons are responsible for rectifier currents, which regulate action potential firing.[393]
It has been suggested that in these high-frequency firing neurons Kv2.2 contributes to maintain action potential amplitude by regulating the interspike potential and by relieving Na+ channels from inactivation [309]
Also regulates spike shape and Ca++ influx during repetitive firing at high frequency in cortical pyramidal neurons [1659]
Kv2 delayed rectified channels are particularly important in the regulation of somatodendritic excitability [175]
SLEEP
Kv2.2 is expressed in approximately 60% of GABAergic neurons and as a result is a viable molecular target to study the functional role of these GABAergic neurons in sleep behaviour [16333]
Insulin and Diabetes
Kv2.2 silencing in mouse islets by adenovirus-small hairpin RNA (shRNA) specifically enhanced islet somatostatin, but not insulin, secretion. In mice lacking somatostatin receptor 5, GxTX-1E stimulated insulin secretion and improved glucose tolerance. Collectively, these data show that Kv2.1 regulates insulin secretion in β-cells and Kv2.2 modulates somatostatin release in δ-cells. Development of selective Kv2.1 inhibitors without cross inhibition of Kv2.2 may provide new avenues to promote GSIS for the treatment of type 2 diabetes [1735]
Noise induced hearing loss
Kv2.2 regulates neuronal excitability in these brainstem nuclei by maintaining short APs and enhancing high-frequency firing. This safeguards efferent MOC firing during high-intensity sounds and is crucial in the mediation of protection after auditory overexposure [1748]
Citalopram
Transfection of HEK293 cells with Kv2.1 or Kv2.2 constructs indicated that citalopram mainly inhibited Kv2.2 current. We suggest that citalopram-induced inhibition of I(K) in mouse cortical neurons is independent of G-protein-coupled receptors and might exert its antidepressant effects by enhancing presynaptic efficiency [1657]
Syntaxin 1A
slowed down inactivation of Kv2.2 (and Kv2.1). No significant changes in current amplitudes were observed in either Kv2.1 or Kv2.2 channels, in the presence of low or high concentrations of syntaxin.[6]
mKvB4
mKvβ4 assayed in the Xenopus oocyte expression system had no effect on Kv1.1, Kv1.2, Kv1.3, Kv1.4, and Kv1.5 α subunits or on the other K+ channel α subunits in the other subfamilies such as Kv3.4 or Kv4.1 or on the K+ channel activity produced by IsK. It specifically altered the expression of the Kv2.2 (CDRK) α subunit [1658]
SNAP-25
Kv2.2 steady-state inactivation was not affected by SNAP-25 alone but (in contrast to Kv2.1), in the presence of both, SNAP-25 and syntaxin 1A, the hyperpolarizing shift of Kv2.2 was apparent.[6]
Tetraethylammonium
The K+ current of Kv2.2 grown in Xenopus oocytes can be stopped with tetraethylammonium (IC50 = 2.6 mM).[278]
4-aminopyridine
The K+ current of Kv2.2 grown in Xenopus oocytes can be stopped with 4-aminopyridine (IC50 = 1.5mM at 120 mV).[278]
Quinine
The K+ current of Kv2.2 grown in Xenopus oocytes can be stopped with quinine (IC50 = 13.7 μM).[278]
Charybdotoxin
Kv2.2 grown in Xenopus oocytes is insensitive to charybdotoxin.[278]
Kv2.1 Co-expression
Co-overexpression of Kv2.1 and Kv2.2 in 832/13 cells impaired the increase in outward K+ current observed with Kv2.1 overexpression alone, and co-immunoprecipitation studies demonstrated a physical interaction between the two proteins. Also, co-overexpression of Kv2.1 and Kv2.2 in β-cells resulted in reduced Kv current compared with overexpression of Kv2.1 alone [1626]
Heteromultimers
To probe whether Kv2 isotypes can form heteromultimers, we developed a dominant-negative mutant Kv2.2 subunit to act as a molecular poison of Kv2 subunit-containing channels. The dominant-negative Kv2.2 suppresses formation of functional channels when it is coexpressed in oocytes with either wild-type Kv2.2 or Kv2.1 subunits. These results indicate that Kv2.1 and Kv2.2 subunits are capable of heteromultimerization. Thus, in native cells either Kv2.1 and Kv2.2 subunits are targeted at an early stage to different biosynthetic compartments or heteromultimerization otherwise is inhibited.
Resveratrol
Resveratrol [trans-3′ 4′,5′-trihydroxystilbene (REV)] is a naturally occurring phytochemical compound that is found in >70 plant species. Plants synthesize REV to protect against bacterial and fungal infections, stress, and injury. Accumulating evidence indicates that REV mediates a wide range of biological activities, including increasing life span through anti-ischemic, anticancer, antiaging, and anti-inflammatory properties. REV significantly suppressed Kv2.2 but not Kv2.1 currents with a fast, reversible, and mildly concentration-dependent manner and shifted the activation or inactivation curve of Kv2.2 channels [1656]
References
Wolf-Goldberg T
et al.
Target soluble N-ethylmaleimide-sensitive factor attachment protein receptors (t-SNAREs) differently regulate activation and inactivation gating of Kv2.2 and Kv2.1: Implications on pancreatic islet cell Kv channels.
Mol. Pharmacol.,
2006
Sep
, 70 (818-28).
Guan D
et al.
Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons.
J. Physiol. (Lond.),
2007
Jun
15
, 581 (941-60).
Schmalz F
et al.
Molecular identification of a component of delayed rectifier current in gastrointestinal smooth muscles.
Am. J. Physiol.,
1998
May
, 274 (G901-11).
Johnston J
et al.
Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in medial nucleus of the trapezoid body neurons.
J. Physiol. (Lond.),
2008
Jul
15
, 586 (3493-509).
Kihira Y
et al.
Formation of heteromeric Kv2 channels in mammalian brain neurons.
J. Biol. Chem.,
2010
May
14
, 285 (15048-55).
Gravagna NG
et al.
Localization of Kv2.2 protein in Xenopus laevis embryos and tadpoles.
J. Comp. Neurol.,
2008
Oct
10
, 510 (508-24).
Hermanstyne TO
et al.
Kv2.2: a novel molecular target to study the role of basal forebrain GABAergic neurons in the sleep-wake cycle.
Sleep,
2013
Dec
, 36 (1839-48).
Lai HC
et al.
The distribution and targeting of neuronal voltage-gated ion channels.
Nat. Rev. Neurosci.,
2006
Jul
, 7 (548-62).
Blaine JT
et al.
Kv2 channels form delayed-rectifier potassium channels in situ.
J. Neurosci.,
2001
Mar
1
, 21 (1473-80).
Jensen MV
et al.
Control of Kv2.2 expression by pyruvate-isocitrate cycling regulates glucose-stimulated insulin secretion.
J. Biol. Chem.,
2013
Jun
20
, ().
Moody WJ
Subtype-specific mechanisms for regulating K+ channel density during development. Focus on "The carboxyl tail region of the Kv2.2 subunit mediates novel developments of channel density".
J. Neurophysiol.,
2004
Dec
, 92 (3169-70).
Blaine JT
et al.
Carboxyl tail region of the Kv2.2 subunit mediates novel developmental regulation of channel density.
J. Neurophysiol.,
2004
Dec
, 92 (3446-54).
Blaine JT
et al.
Heteromultimeric potassium channels formed by members of the Kv2 subfamily.
J. Neurosci.,
1998
Dec
1
, 18 (9585-93).
Dong WH
et al.
Resveratrol inhibits K(v)2.2 currents through the estrogen receptor GPR30-mediated PKC pathway.
Am. J. Physiol., Cell Physiol.,
2013
Sep
, 305 (C547-57).
Zhan XQ
et al.
The antidepressant citalopram inhibits delayed rectifier outward K⁺ current in mouse cortical neurons.
J. Neurosci. Res.,
2012
Jan
, 90 (324-36).
Fink M
et al.
A new K+ channel beta subunit to specifically enhance Kv2.2 (CDRK) expression.
J. Biol. Chem.,
1996
Oct
18
, 271 (26341-8).
Du J
et al.
Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1.
J. Physiol. (Lond.),
2000
Jan
1
, 522 Pt 1 (19-31).
Li XN
et al.
The role of voltage-gated potassium channels Kv2.1 and Kv2.2 in the regulation of insulin and somatostatin release from pancreatic islets.
J. Pharmacol. Exp. Ther.,
2013
Feb
, 344 (407-16).
Hermanstyne TO
et al.
Immunolocalization of the voltage-gated potassium channel Kv2.2 in GABAergic neurons in the basal forebrain of rats and mice.
J. Comp. Neurol.,
2010
Nov
1
, 518 (4298-310).
Tong H
et al.
Protection from noise-induced hearing loss by Kv2.2 potassium currents in the central medial olivocochlear system.
J. Neurosci.,
2013
May
22
, 33 (9113-21).
Khazen G
et al.
Combinatorial Expression Rules of Ion Channel Genes in Juvenile Rat (Rattus norvegicus) Neocortical Neurons.
PLoS ONE,
2012
, 7 (e34786).
Bishop HI
et al.
Distinct Cell- and Layer-Specific Expression Patterns and Independent Regulation of Kv2 Channel Subtypes in Cortical Pyramidal Neurons.
J. Neurosci.,
2015
Nov
4
, 35 (14922-42).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/10/ , accessed on 2024 May 09