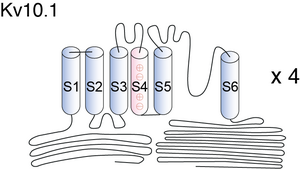
Kv10.1
Description: potassium voltage-gated channel, subfamily H (eag-related), member 1 Gene: Kcnh1 Alias: Kv10.1, EAG, EAG1, h-eag, MGC142269, KCNH1
Kv10.1, encoded by the KCNH1, is a potassium channel, voltage-gated, subfamily H. It is expressed almost exclusively in brain tissue and is involved in cell excitability, memory processes, cell proliferation, and tumour progression [1509]
Experimental data
Rat Kv10.1 gene in CHO host cells datasheet |
||
|
Click for details 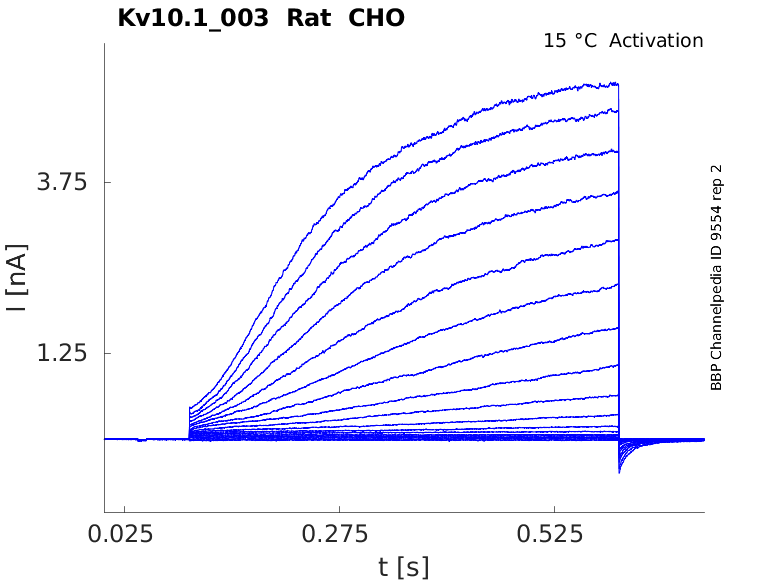
15 °Cshow 70 cells |
Click for details 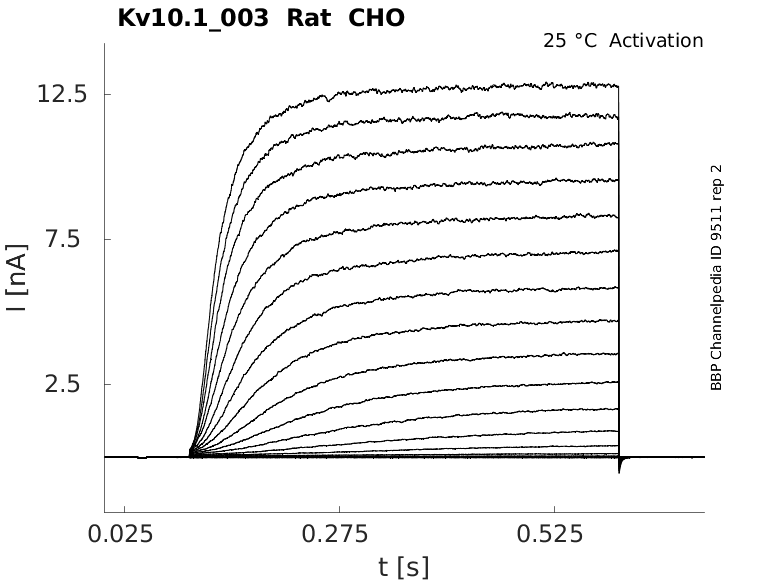
25 °Cshow 65 cells |
Click for details 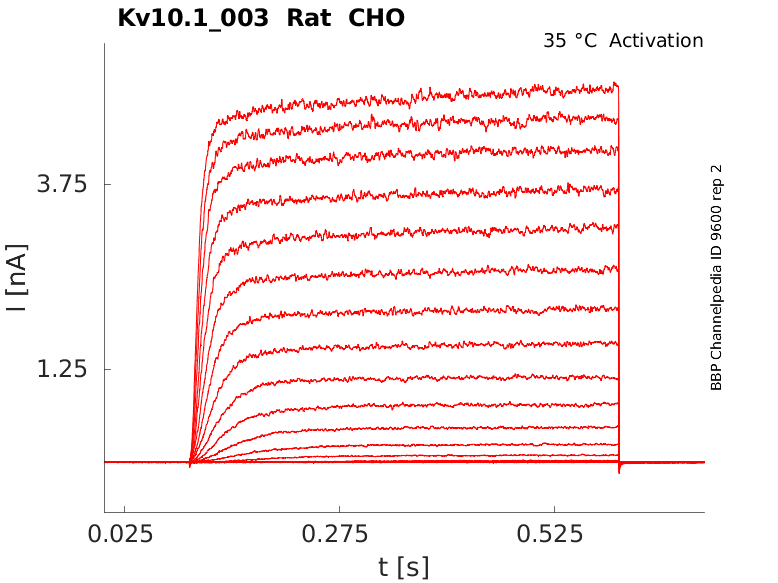
35 °Cshow 80 cells |
Mouse Kv10.1 gene in CHO host cells |
||
|
Click for details 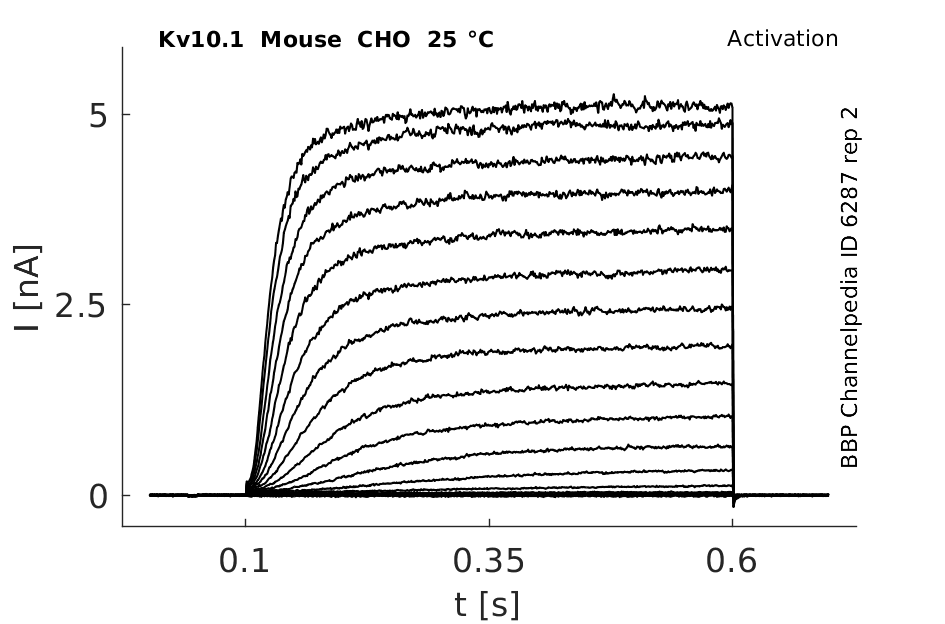
25 °Cshow 42 cells |
||
Rat Kv10.1 gene in HEK host cells |
||
|
Click for details 
25 °Cshow 25 cells |
||
Rat Kv10.1 gene in CV1 host cells |
||
|
Click for details 
25 °Cshow 60 cells |
||
Kv10.1 belongs to the ether-à-go-go (EAG) family within the voltage-gated potassium (Kv) channel superfamily.
Human and rat Kv10.1a and b cDNAs encode silent K+ channel pore-forming subunits that modify the electrophysiological properties of Kv2.1. These alternatively spliced variants arise by the usage of an alternative site of splicing in exon 1 producing an 11-amino acid insertion in the linker between the first and second transmembrane domains in Kv10.1b. [675].
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_172362.3 | 8140 | |
| Mouse | NM_010600.3 | 7269 | |
| Rat | NM_031742.2 | 4560 |
Isoforms
Post-Translational Modifications
Visual Representation of Kv10.1 Structure
Methodology for visual representation of structure available here
Crystal Structure of EAG (Kv10.1) channel

Structure of EAG1 ion Channel
Structurally, Eag family are similar to other voltage-gated potassium channels, comprising of four identical α subunits each consisting of six membrane spanning domains (S1-S6) with cytoplasmic amino (N) and carboxy (C) termini. Pore region (P) is positioned between S5-S6. Chain of + arginine or lysine is separated by two hydrophobic residues within S4, this is where voltage is sensed. The N terminal consists of a Per-Arnt-Sim (PAS) domain, a hypoxia sensor leading to the activation of hypoxia inducible factor (HIF1), resulting in increased glycolysis and angiogenesis. The C terminus consists of a cyclic nucleotide binding domain (cNBD) and tetramerization-coil-coil domain with an Endoplasmic reticulum retention signal (RXR), which is involved in the tetramerization and functional expression. Also present on the C terminus are multiple signalling modules including putative nuclear export sequences (NES) and nuclear localization sequences (NLS) with binding sites for calmodulin (CaM), calcium/CaM-dependent protein kinaseII (CaMKII). These NES and NLS play an important role in perinuclear localization of these channels [1505]
N and C terminus
The ether-a-go-go family, named after the Drosophila prototype, is characterized by long N- and C-terminal intracellular tails [81]
Importance of PAS domain
However, for chimera I, which had the first 137 amino acids of heag2 replaced by the corresponding amino acids of heag1, the resulting construct was fast-activating, like heag1. This suggests that these N-terminal residues, which include the PAS domain, play a role in determining the differences in activation kinetics between the heag channels. Furthermore, for chimera II with residues 138–549 of the heag2 channel replaced by corresponding residues from heag1, the activation kinetics were again fast, like heag1. This indicates that the central, membrane- spanning region also plays a role in determining differ- ences in activation kinetics between the two channels [81]
Kv10.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Schematic Drawing of EAG currents

Intenral Na+ Concentration
Human EAG in X oocytes Kinetics

High potassium content
Although the rate of C-type inactivation in Kv channels is slowed by high [K+]e, the rate of Y464A hEAG1 channel inactivation was unaffected by elevation of [K+]e from 2 to 20 mM, and slightly faster at 104 mM [1504]
Markov Model
Markov modelling demonstrated that simple 5- and 6-state models were inadequate and that a 10- or 12-state model was more appropriate for description of EAG1 channels [1504]
Hodgkin and Huxley Model for rEAG

EAG expression in Human
In human, the Kv10s mRNA were detected by Northern blot in brain kidney lung and pancreas. In brain, they were expressed in cortex, hippocampus, caudate, putamen, amygdala and weakly in substantia nigra [675]
EAG expression in Rat
In rat, Kv10.1 products were detected in brain and weakly in testes. In situ hybridization in rat brain shows that Kv10.1 mRNAs are expressed in cortex, olfactory cortical structures, basal ganglia/striatal structures, hippocampus and in many nuclei of the amygdala complex. [675]
It is also expressed in proximal regions of the extensions in human brains [1505]
Retina and Cochlea
Although eag subunits are specifically expressed in the adult rat brain, there are no reports about eag-mediated currents in normal neuro- nal tissue and the physiological role of eag in the central nervous system has still to be elucidated. However, there are indications that eag currents may be present in the retina as described below, and recently, eag1 mRNA has been found to be expressed in certain cells of the cochlea
Cancer
Increased expression of Eag-1 channels is found in several cancer types including breast cancer [768], [769].
EAG Distribution in Neuron
A population of eag antennal sensory neurons appears to be insensitive to a subset of odorants. Is this mutant phenotype caused by a defect in a signal transduction component (Eag channel subunits)? In support of this, Eag K+ channels appear to be located on the outer dendrites of antennal neurons [1722]
EAG1 channels (kv10.1) are very noticeable in the perinuclear space of cells [1505]
Cell Proliferation
These data together with the cell cycle-dependent expression of eag channels, suggest a direct involvement of eag channels in cell proliferation and prompt the possibility that eag channels participate in the transformation of normal cells into tumor cells. This idea was supported by the result that transfection of CHO cells with eag channels led to an increased proliferation rate [1721]
It has been shown that Eag-1 K+ channels, a member of the voltage-activated K+ channel superfamily, play a role in controlling the proliferation and transformation of epithelial cells [766], [767].
Along with BKCa, hEag1 channels not only regulate cell proliferation, but also participate in the adipogenic and osteogenic differentiations in human MSCs (human bone marrow-derived mesenchymal stem cells) [1512]
Channel trafficking
Silencing of Rabaptin-5 induces down-regulation of recycling of Kv10.1 channel in transfected cells and reduction of Kv10.1 current density in cells natively expressing Kv10.1, indicating a role of Rabaptin-5 in channel trafficking [1513]
Kv10.1 possesses oncogenic properties: ether-à-go-go type 1 (Eag1). [767]
Eag channels are expressed in fusing myoblasts and been posulated to have a role in their hyperpolarisation that preceeds their fusion [1507].
Photoreceptors
Eag channels are also involved in odour transduction and are encoded in seizure locus in Drosophila. In mammals, although Eag channels have been shown to be present in rat brain, their exact physiological function is not known, but in rat retina, they are known to be involved in the dark current-loop of photoreceptors [1505].
K/O mice
Because in mammals no physiological function has been reported so far, Kv10.1-deficient mice were experimented on to elucidate the functional role of Kv10.1 in the brain. However, successfully generated Kv10.1-deficient mice displayed no obvious differences from their littermate controls in the average life span, reproductive characteristics or development [1511]
Cancer
The Eag1 K(+) channel might be involved in the pathophysiological processes of prostate cancer, and is expected to be a valuable target for the diagnosis and treatment of prostate cancer [1514]
Tumour
KV10.1 potassium channels are implicated in a variety of cellular processes including cell proliferation and tumour progression. Their expression in over 70% of human tumours makes them an attractive diagnostic and therapeutic target [1509]
Kv2.1
Human and rat Kv10.1a and b cDNAs encode silent K+ channel pore-forming subunits that modify the electrophysiological properties of Kv2.1. Co-injection of Kv2.1 and Kv10.1a or b mRNAs in Xenopus oocytes produced smaller currents that in the Kv2.1 injected oocytes and a moderate reduction of the inactivation rate without any appreciable change in recovery from inactivation or voltage dependence of activation or inactivation [675]
Magnesium
Activation kinetics depend on the holding potential and the extracellular Mg eag1-like membrane currents were elicited in a human neuroblastoma cell in response to depolarizations to 50 mV from holding potentials which varied from −130 to −40 mV in steps of 10 mV in external solutions containing the indicated concentrations of Mg2+ or 50
Calcium induces block in CHO cells

Hyperkinetic B-subunit
So far, a few B-subunits have been found to interact with eag1 channels and to affect their biophysical properties. The Drosophila B-subunit hyperkinetic increases the current amplitude mediated by Drosophila eag1 chan- nels, slightly accelerates current activation and slows inactivation [1717]
EPSIN
The protein epsin was isolated from rat brain and has been found to bind to eag1 channels, thereby decreasing the probability of channel opening at potentials near the resting potential [1718]
KCR1
Binding of the protein KCR1 to eag channels leads to an increase in the eag current amplitude by induction of a faster eag channel activation and a shift of the activation curve by 10 mV to more negative membrane potentials [1719]
ICA

Mutation
Mutation of Tyr652 in S6 segment enhances hEAG1 channel inactivation [1504]
References
Wray D
The roles of intracellular regions in the activation of voltage-dependent potassium channels.
Eur. Biophys. J.,
2004
May
, 33 (194-200).
Ottschytsch N
et al.
Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome.
Proc. Natl. Acad. Sci. U.S.A.,
2002
Jun
11
, 99 (7986-91).
Vega-Saenz de Miera EC
Modification of Kv2.1 K+ currents by the silent Kv10 subunits.
Brain Res. Mol. Brain Res.,
2004
Apr
7
, 123 (91-103).
Borowiec AS
et al.
Regulation of IGF-1-dependent cyclin D1 and E expression by hEag1 channels in MCF-7 cells: the critical role of hEag1 channels in G1 phase progression.
Biochim. Biophys. Acta,
2011
May
, 1813 (723-30).
Ouadid-Ahidouch H
et al.
K+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis.
J. Membr. Biol.,
2008
Jan
, 221 (1-6).
Pardo LA
et al.
Role of voltage-gated potassium channels in cancer.
J. Membr. Biol.,
2005
Jun
, 205 (115-24).
Hemmerlein B
et al.
Overexpression of Eag1 potassium channels in clinical tumours.
Mol. Cancer,
2006
, 5 (41).
Mello de Queiroz F
et al.
Ether à go-go potassium channel expression in soft tissue sarcoma patients.
Mol. Cancer,
2006
, 5 (42).
Garg V
et al.
Tuning of EAG K(+) channel inactivation: molecular determinants of amplification by mutations and a small molecule.
J. Gen. Physiol.,
2012
Sep
, 140 (307-24).
Asher V
et al.
Eag and HERG potassium channels as novel therapeutic targets in cancer.
,
2010
Dec
29
, 8 (113).
Hammadi M
et al.
Human ether à-gogo K(+) channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry.
J. Cell. Physiol.,
2012
Dec
, 227 (3837-46).
Occhiodoro T
et al.
Cloning of a human ether-a-go-go potassium channel expressed in myoblasts at the onset of fusion.
FEBS Lett.,
1998
Aug
28
, 434 (177-82).
Jiménez-Garduño AM
et al.
KV10.1 K(+)-channel plasma membrane discrete domain partitioning and its functional correlation in neurons.
Biochim. Biophys. Acta,
2014
Mar
, 1838 (921-31).
Gerlach AC
et al.
Pharmacological removal of human ether-à-go-go-related gene potassium channel inactivation by 3-nitro-N-(4-phenoxyphenyl) benzamide (ICA-105574).
Mol. Pharmacol.,
2010
Jan
, 77 (58-68).
Ufartes R
et al.
Behavioural and functional characterization of Kv10.1 (Eag1) knockout mice.
Hum. Mol. Genet.,
2013
Jun
1
, 22 (2247-62).
Zhang YY
et al.
BKCa and hEag1 channels regulate cell proliferation and differentiation in human bone marrow-derived mesenchymal stem cells.
J. Cell. Physiol.,
2013
Jul
23
, ().
Ninkovic M
et al.
Physical and functional interaction of K(V)10.1 with Rabaptin-5 impacts ion channel trafficking.
FEBS Lett.,
2012
Jul
25
, ().
Zheng YQ
et al.
[Expression of Eag1 K(+) channel in prostate cancer and its significance].
Zhonghua Nan Ke Xue,
2013
Mar
, 19 (205-9).
Chae YJ
et al.
Escitalopram block of hERG potassium channels.
Naunyn Schmiedebergs Arch. Pharmacol.,
2014
Jan
, 387 (23-32).
Camacho J
et al.
Cytoskeletal interactions determine the electrophysiological properties of human EAG potassium channels.
Pflugers Arch.,
2000
Dec
, 441 (167-74).
Wilson GF
et al.
Interaction of the K channel beta subunit, Hyperkinetic, with eag family members.
J. Biol. Chem.,
1998
Mar
13
, 273 (6389-94).
Piros ET
et al.
Purification of an EH domain-binding protein from rat brain that modulates the gating of the rat ether-à-go-go channel.
J. Biol. Chem.,
1999
Nov
19
, 274 (33677-83).
Hoshi N
et al.
KCR1, a membrane protein that facilitates functional expression of non-inactivating K+ currents associates with rat EAG voltage-dependent K+ channels.
J. Biol. Chem.,
1998
Sep
4
, 273 (23080-5).
Lecain E
et al.
Potassium channel ether à go-go mRNA expression in the spiral ligament of the rat.
Hear. Res.,
1999
Jul
, 133 (133-8).
Pardo LA
et al.
Cell cycle-related changes in the conducting properties of r-eag K+ channels.
J. Cell Biol.,
1998
Nov
2
, 143 (767-75).
Dubin AE
et al.
The K+ channel gene ether a go-go is required for the transduction of a subset of odorants in adult Drosophila melanogaster.
J. Neurosci.,
1998
Aug
1
, 18 (5603-13).
Terlau H
et al.
Amino terminal-dependent gating of the potassium channel rat eag is compensated by a mutation in the S4 segment.
J. Physiol. (Lond.),
1997
Aug
1
, 502 ( Pt 3) (537-43).
Haitin Y
et al.
The structural mechanism of KCNH-channel regulation by the eag domain.
Nature,
2013
Sep
19
, 501 (444-8).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/33/ , accessed on 2024 May 08