Kv9.1
Description: potassium voltage-gated channel, delayed-rectifier, subfamily S, member 1 Gene: Kcns1 Alias: Kv9.1, kcns1
Kv9.1, encoded by the gene JCNS1, is a member 1 of subfamily S of potassium voltage-gated delayed-rectifier channels. Kv9.1 is not functional by itself but can form heteromultimers with member 1 and with member 2 of the Shab-related subfamily of potassium voltage-gated channel proteins. NCBI
Experimental data
Rat Kv9.1 gene in CHO host cells datasheet |
||
|
Click for details 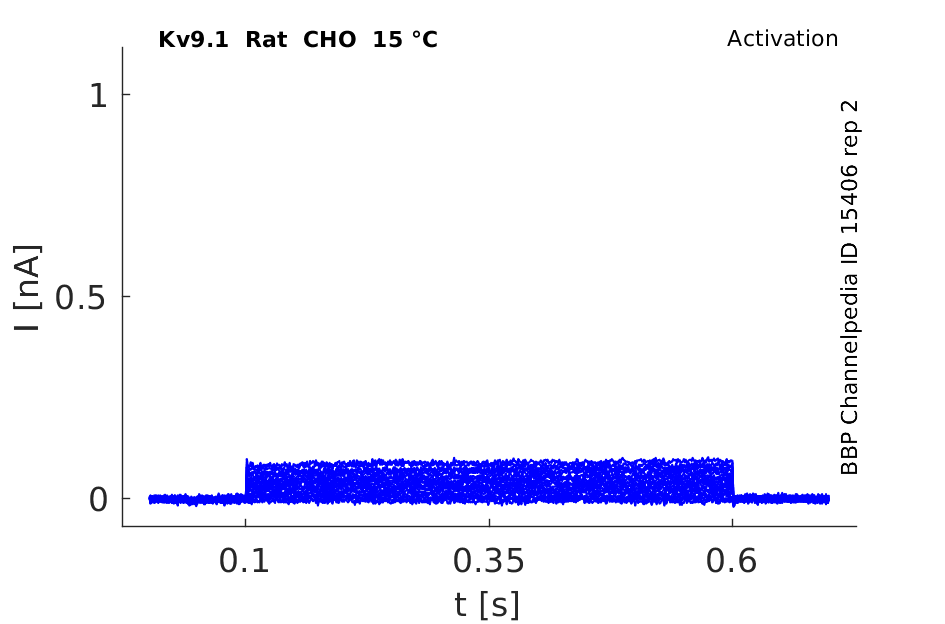
15 °Cshow 62 cells |
Click for details 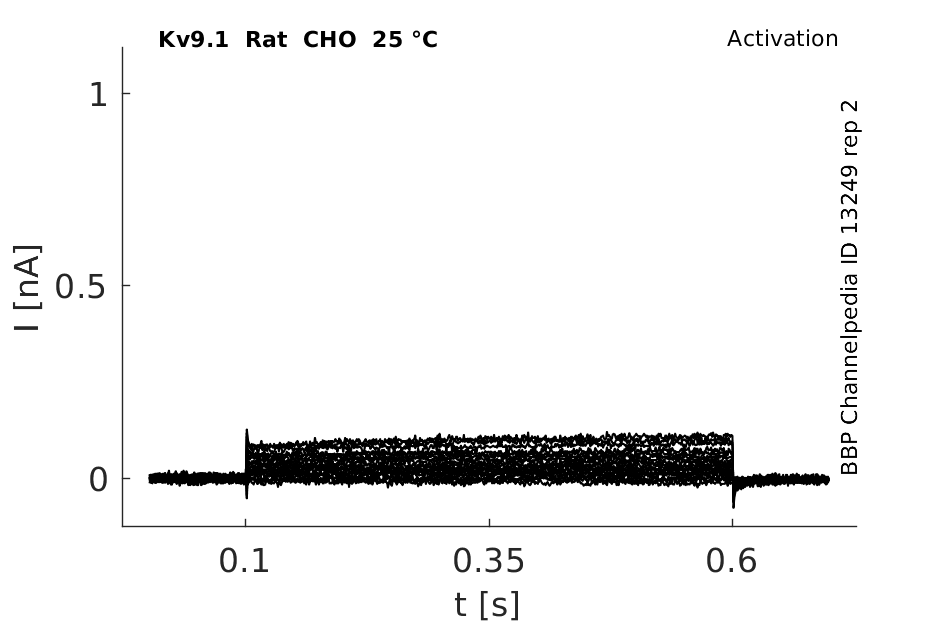
25 °Cshow 64 cells |
Click for details 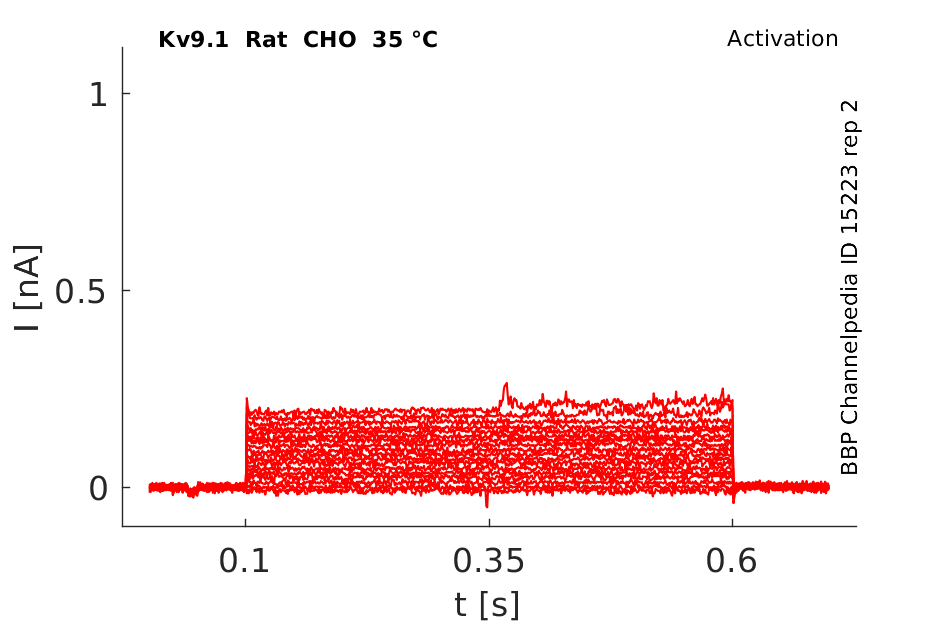
35 °Cshow 83 cells |
In contrast to the genes in the other subfamilies, members of the Kv8 and Kv9 subfamilies have been found to be incapable of forming functional channels when expressed either in oocytes or cell lines. Moreover, these mammalian genes appear to have no Drosophila homologs. [402]
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_002251.5 | 5649 | |
| Mouse | NM_008435.2 | 2727 | |
| Rat | NM_053954.1 | 2720 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv9.1 Structure
Methodology for visual representation of structure available here
Kv9.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Kv9.1 Kinetics affect Kv2.2 and Kv1.3

Kv9.1 interacts with Kv2.1 in CHO cells
In our experiments, both hKv9.1 and hKv9.3 slowed the deactivation and inactivation of hKv2.1-dependent currents, in keeping with previous results (18, 26). Interestingly, with the cotransfection of either electrically silent subunit with hKv2.1, inactivation and deactivation time courses more closely matched those measured from native human lens cells than did the time courses measured from hKv2.1 alone. Transfection with either hKv2.1-hKv9.1 or hKv2.1-hKv9.3 fusion proteins produces about the same slowing of activation as cotransfection of unfused subunits [1795]
Expression of Kv9.1 in DRG sections of Rats
We first examined the expression of Kv9.1 transcripts in lumbar DRG sections of naive rats, using ISH. Size-frequency analysis revealed that Kv9.1 mRNA was robustly expressed in medium-large (>30 μm diameter, 89.7 ± 1.5%), but not small (<30 μm diameter, 5.6 ± 1.4%) neurons [1755]
Kv9.1 and Kv9.2, two known members of the Kv9 subfamily, appear to be expressed diffusely in cells of the inferior colliculus [402].
Kv9.1 Expression in Rat Brain
High levels of expressions are found in the olfactory bulb, cerebral cortex, hippocampal formation, habenula, basolateral amygdaloid nuclei, and cerebellum. Interestingly Kv9.1 and Kv9.2 colocalized with Kv2.1 and/or Kv2.2 α subunits in several regions of the brain [400]
Both Kv9.1 and Kv9.2 are neuronal modulatory alpha subunits that define a structural family designated as Kv9. They modulate Kv2.alpha subunits but have no functional activity by themselves. [400]
Inhibitor of Neuronal Excitability
Our study is the first one to address the effect of Kv9.1 in vivo and our results strongly suggest that, in sensory neurons, Kv9.1 is an inhibitor of neuronal excitability [1775]. Interestingly, Kv9.1 is not a pore-forming subunit and therefore its influence on sensory neuron excitability must be indirect [400]
K+ channel functions are included in very diverse processes such as neuronal integration, cardiac pacemaking, muscle contraction, and hormone secretion in ex- citable cells [735], as well as in cell proliferation, cell volume regulation, and lymphocyte differentiation [736].
Kv9.1 and Kv9.2 modulate Kv2.alpha subunits but have no functional activity by themselves [400]. I.e., the kinetics of activation and the voltage-dependence of Kv2.1 currents are altered by such co-expression with Kv9.1 [402].
Hyper excitability
Of particular interest, we illustrate that Kv9.1 dysfunction can also trigger a form of stimulus-dependent hyperexcitability in sensory neurons. This firing did not depend on any CNS connection as it was observed in axons that were centrally disconnected [1775]
Neuropathic Pain
Our results propose that Kv9.1 downregulation after nerve injury may be the molecular switch controlling myelinated sensory neuron hyperexcitability. Intriguingly, a recent wide-genome association screen in humans identified a Kv9.1 polymorphism associated with susceptibility to develop chronic neuropathic pain after back surgery or leg amputation, suggesting that the mechanisms described in our studies will be of direct clinical relevance to human pain [1775] [1794]
Kv2.1 and Kv2.2 Co-assembly
Since previous studies have suggested that Kv9.1 function is linked to coassembly with Kv2.1 and Kv2.2 subunits, we investigated whether Kv9.1 and Kv2 subunits are coexpressed in DRG neurons. To achieve this, we performed ISH for Kv9.1/Kv2.1 (left) and Kv9.1/Kv2.2 (right) in adjacent DRG sections and were able to directly observe colocalization of these mRNAs in single medium-large neurons (arrows). Examining the Kv9.1-positive population, we found that 48.6 and 76.9% of cells coexpressed Kv2.1 or Kv2.2, respectively. The presence of both Kv2 subunits was documented in more than a third of all Kv9.1-positive neurons (36.7%), while only a small number did not express any Kv2 subunit (11.25%) [1775]
The Kv9.1 gene encodes a potassium channel alpha subunit that is expressed in a variety of neurons, including those of the inferior colliculus. When cRNA encoding this subunit is injected into Xenopus oocytes, no functional channels are expressed. Kv9.1 isolated from a rat brain cDNA library alters the kinetics and the voltage-dependence of activation and inactivation of Kv2.1, a channel subunit that generates slowly inactivating delayed rectifier potassium currents. The rate of activation of Kv2.1 is slowed by co-expression with Kv9.1. With Kv2.1 alone, the amplitude of evoked currents increases monotonically with increasing command potentials. In contrast, when Kv2.1 is co-expressed with Kv9.1, the amplitude of currents increases with increasing depolarization up to potentials of only ∼+60 mV, after which increasing depolarization results in a decrease in current amplitude. [402]
References
Salinas M
et al.
New modulatory alpha subunits for mammalian Shab K+ channels.
J. Biol. Chem.,
1997
Sep
26
, 272 (24371-9).
Stocker M
et al.
Cloning and tissue distribution of two new potassium channel alpha-subunits from rat brain.
Biochem. Biophys. Res. Commun.,
1998
Jul
30
, 248 (927-34).
Richardson FC
et al.
Modification of delayed rectifier potassium currents by the Kv9.1 potassium channel subunit.
Hear. Res.,
2000
Sep
, 147 (21-30).
Sheng M
et al.
Presynaptic A-current based on heteromultimeric K+ channels detected in vivo.
Nature,
1993
Sep
2
, 365 (72-5).
Wang H
et al.
Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons.
Nature,
1993
Sep
2
, 365 (75-9).
Suh BC
et al.
Modulation of high-voltage activated Ca(2+) channels by membrane phosphatidylinositol 4,5-bisphosphate.
Neuron,
2010
Jul
29
, 67 (224-38).
Lewis RS
et al.
Potassium and calcium channels in lymphocytes.
Annu. Rev. Immunol.,
1995
, 13 (623-53).
Pongs O
Structure-function studies on the pore of potassium channels.
J. Membr. Biol.,
1993
Oct
, 136 (1-8).
Heginbotham L
et al.
Mutations in the K+ channel signature sequence.
Biophys. J.,
1994
Apr
, 66 (1061-7).
MacKinnon R
Pore loops: an emerging theme in ion channel structure.
Neuron,
1995
May
, 14 (889-92).
Pascual JM
et al.
K+ pore structure revealed by reporter cysteines at inner and outer surfaces.
Neuron,
1995
May
, 14 (1055-63).
Christie MJ
et al.
Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits.
Neuron,
1990
Mar
, 4 (405-11).
Shahidullah M
et al.
Slow inactivation conserved in heteromultimeric voltage-dependent K+ channels between Shaker (Kv1) and Shaw (Kv3) subfamilies.
FEBS Lett.,
1995
Sep
11
, 371 (307-10).
Chen ML
et al.
Heteromultimeric interactions among K+ channel subunits from Shaker and eag families in Xenopus oocytes.
Neuron,
1996
Sep
, 17 (535-42).
Tsantoulas C
et al.
Sensory neuron downregulation of the Kv9.1 potassium channel subunit mediates neuropathic pain following nerve injury.
J. Neurosci.,
2012
Nov
28
, 32 (17502-13).
Costigan M
et al.
Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1.
Brain,
2010
Sep
, 133 (2519-27).
Shepard AR
et al.
Electrically silent potassium channel subunits from human lens epithelium.
Am. J. Physiol.,
1999
Sep
, 277 (C412-24).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/30/ , accessed on 2024 May 08