Nav1.9
Description: sodium channel, voltage-gated, type XI, alpha Gene: Scn11a Alias: nav3.1, nav1.9, scn11a
Nav1.9 (also known as NaN; SNS-2; NAV1.9; scn12a), encoded by the gene scn11a, is a sodium, voltage-gated, type 11, alpha subunit channel. Nav1.9 is predominantly expressed in the PNS. It is involved in pain-related signaling and serves as a threshold channel. Mutations to the Nav1.9 have been linked to pain disorders.
scn11a, the gene encoding for Nav1.9, is located on chromosome 3 in humans, more specifically at the position 3p21-24. The channel is made up of 30 exons, 26 of which are coding and exons 1 to 4 being non-coding.
Other tetrodotoxin-resistant channels such as Nav1.5 and Nav1.8, encoded respectively by scn5a and scn10a respectively, are also present within this same gene region, suggesting a possible common ancestral gene or evolutionary link. All 3 genes contain an extra exon (17b) between exons 17 and 18, which corresponds to a section on the loop between domain II and domain III. [2123]
There exists various confirmed and predicted mRNA variants of Nav1.9 , with variant 1 is shown as the main form in most databases
The Nav1.9 mRNA expression starts off low and gradually increases over embryonic development, to reach stable levels post-birth to adulthood [2124].
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_014139.3 | 6505 | |
| Mouse | NM_011887.3 | 5837 | |
| Rat | NM_019265.2 | 5905 |
Human Nav1.9 is composed of 1792 amino acids compared to 1765 residues in mice and rats [1439]. The protein has a molecular weight of 205 Kda. Slight variations in amino acids between species has been shown to impact the kinetic properties of Nav1.9. For example, arginine is present in the fourth subunit of domain two in humans (D2/S4), whereas alanine is present within rats and mice. This results in an observed 10.20 mV depolarising shift in activation [1439].
There exists a number of protein isoforms that arise from the translation of the aforementioned transcript variants though many of these isoforms have not been extensively characterised
Isoforms
Like most mammalian proteins, Nav1.9 is subject to a series of post translational modifications (PTM).
Phosphorylation via the activity of protein kinases (PKA, PKC, MAPK, etc.) leads to an increase in Nav1.9 current [2128].
Nav1.9 can also be glycosylated, with some patterns being developmentally regulated [2129].
Nav1.9 is generally not ubiquitinated as it does not contain a PY motif (PPXY), or any similar motif variants (LPXY), that interacts with NEDD4-2, an enzyme responsible for ubiquitination [2130].
Visual Representation of Nav1.9 Structure
Methodology for visual representation of structure available here
Like all voltage gated sodium channels, Nav1.9 is made up of a single protein comprised of 4 homologous domains (DI-DIV). Each domain is made up of 6 transmembrane subunits (S1-S6). S1-4 form the voltage sensing domain (VSD) whereas the S5-6 form the pore module (PM). The S4 subunit of each domain contains a series of positively charged residues. When membrane depolarization occurs, these charged residues cause the movement of the S4 subunit, inducing a conformational change in S5-S6, opening of the channel and allowing the entry of sodium ions into the cell. Soon after opening, rapid inactivation of Nav1.9 is instigated by the binding of the IFM motif, found in the loop between D3 and D4, to a hydrophobic receptor site next to the S6 in D4. This binding causes the shift of S6, allosterically closing the channel, thus deactivating the channel. Nav1.9 then returns to its resting state following the hyperpolarization of the cell membrane [2115].
Nav1.9 has a serine residue at position 335 within the pore-lining section of domain 1 (Ser335; D1/S2). The presence of serine in this area, as opposed to an amino acid with an aromatic ring, reduces the affinity of tetrodotoxin (TTX) to the ion channel, therefore making Nav1.9 TTX resistant [2123]. Other sections of the protein are also important for the proper function of the channel. For example, Nav1.9 has been shown to contain a number of predicted phosphorylation sites in the intracellular loops and N-glycosylation sites in the extracellular linkers [899]. Another important segment is the C terminus, which has been shown to contain a 49-aa motif which regulates Nav1.9 trafficking to the plasma membrane [2131].
The approximative size/surface of Nav1.9 can be determined via the resolved or predicted structures.
Nav1.9 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
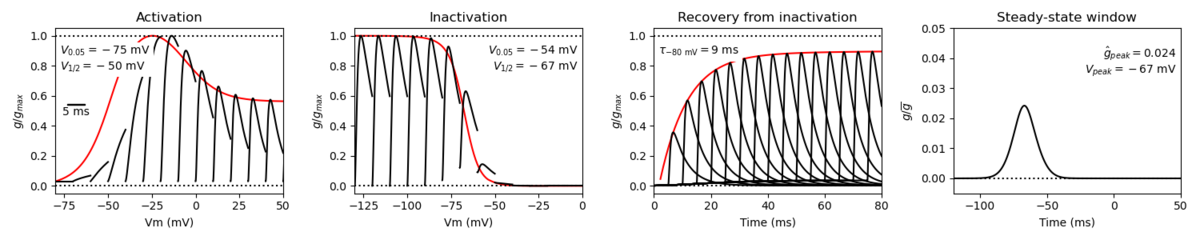
Nav1.9 has some unique kinetic properties and mediates a TTX-Resistant current.
Nav1.9 activates at more hyperpolarized voltages than other voltage gated sodium channels. The channel inactivation is characterized as “ultra-slow” and results in the persistence of current post inactivation. The large voltage overlap between activation and inactivation creates a window current, whereby there exists a wide range of voltages at which the channel is open [2123].
Single Channel Unitary Conductance
Single channel unitary conductance is determined experimentally.
For Nav1.9, single channel unitary conductance has yet to be determined experimentally.
Model
A single kinetic model for all human voltage-gated sodium channels (Balbi et al, 2017)
https://modeldb.science/230137
Species : Human | Gene: scn11a
Host cell : HEK293 cells | Temperature: RT (to 25 C by Q10)
Formalism: Markov | States: C1, C2, O1, O2, I1, I2
Implementation: NEURON | Simulation (Nav16_a.mod)
Tissue and Cellular
Nav1.9 is found primarily in the peripheral nervous system (PNS) in functionally identified nociceptors.
Nav1.9 is expressed in small diameter (<30 μm) DRG neurons, in trigeminal ganglion neurons, substantia gelatinosa of Rolando (SGR), and in intrinsic myenteric neurons. Among the small diameter neurons, Nav1.9 is preferentially expressed in the somatosensory non-peptidergic DRG neurons.
Nav1.9 has also been found in the dorsal horn but at significantly lower expression levels [2123].
On a subcellular level, NaV1.9 has been localized predominantly within DRG neuronal somata but it can also be found in the free nerve terminals and at central terminals within the outer layers of the SGR [2123].
Threshold Channel
Given its biophysical properties (“slow” kinetics and large window current) Nav1.9 is not thought to contribute to the generation or amplitude of the action potential. Instead, Nav1.9 acts as a threshold channel. Through the persistent current that it generates, Nav1.9 is thought to regulate membrane potential and prolong the depolarization response to subthreshold stimuli. This results in the lowering of the depolarisation threshold necessary for the generation of action potentials and repetitive firing [2123].
Nociception & Channelopathies
Nav1.9 localisation in nociceptors serves as primary evidence in its role in pain signaling. Further evidence of the channel’s involvement in pain sensation is highlighted by the various channelopathies that arise as a result of mutations in the coding gene scn11a.
Multiple studies have reported a number of scn11a variants which result in gain of function mutations, with individuals carrying the mutation experiencing increased pain. The mutant channels had increased excitability and thus increased the downstream generation of action potentials and firing [2132] [2133] [2134].
Conversely, another scn11a mutation was identified which resulted in the patient’s inability to experience pain. Interestingly, this mutation, L811P, still resulted in a gain of function mutation. Nav1.9 channels showed a slowed deactivation and strongly hyperpolarized voltage dependence of activation, suggesting that mutated Nav1.9 are hyperexcitable. The exact reasons as to why gain-of-function mutations induce both pain sensitivity and insensitivity have yet to be determined [2135] [2136]. Regardless, such mutations help illustrate the fundamental role of Nav1.9 in proper nociception.
Nav1.9 involvement in other types of pain have also been studied. Given its interaction with inflammatory mediators, Nav1.9 has been shown to play a role in inflammatory pain, with increased channel and neuron excitability after the release of inflammatory compounds post injury [2123]. Multiple studies have showcased the role of Nav1.9 in pain signaling within gastrointestinal diseases [2137].
There are several molecules whose interaction with Nav1.9 leads to an increase ion channel currents within DRG neurons:
- Inflammatory mediators (PGE2, serotonin, bradykinin, histamine, PGE3 and norepinephrine [223] [1440].
- Secreted proteins that augment neuronal survival (BDNF, GDNF)[1438] [1442].
It is worth noting that any modifications in the pathways of the aforementioned molecules, via the interaction of other compounds, will also have a subsequent impact on the activity of Nav1.9. Therefore the targeting of molecules upstream of Nav1.9 could serve as potential ways to modulate the activity of Nav1.9 [2138] [2139].
Other interacting molecules influence the trafficking and subcellular distribution of Nav1.9.
Contactin is thought to interact with Nav1.9 and influence the surface localisation of the ion channel as coexpression experiments of both protein has been shown to increase the number of Nav1.9 channels at the cell surface [2125].
Interaction with the subunit Navβ1 is essential for the proper activity of Nav1.9. Navβ1 deficient DRG neurons exhibit a depolarizing shift in the voltage dependence inactivation, reduced persistent current, a prolonged rate of recovery from inactivation, and reduced cell surface expression of Nav1.9 compared to its wild-type counterparts [2126]. Other experiments, expressing Nav1.9 constructs in HEK-293 Cells achieved highest current densities when co-expressed with Navβ1 and Navβ2, suggesting the possible interaction with Navβ2 for proper function [2127]
Despite numerous interactions documented, no Nav1.9-specific drugs have been to date identified, with further research is still necessary to uncover Nav1.9 specific interaction. However, a number of non-specific sodium channel blockers have been shown to modulate the activity of Nav1.9.
For additional resources on potential drug and compound interactions:
References
Rush AM
et al.
PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins.
Brain Res.,
2004
Oct
15
, 1023 (264-71).
Catterall WA
From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels.
Neuron,
2000
Apr
, 26 (13-25).
Catterall WA
et al.
International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels.
Pharmacol. Rev.,
2005
Dec
, 57 (397-409).
Cummins TR
et al.
A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons.
J. Neurosci.,
1999
Dec
15
, 19 (RC43).
Dib-Hajj SD
et al.
NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy.
Proc. Natl. Acad. Sci. U.S.A.,
1998
Jul
21
, 95 (8963-8).
Delmas P
et al.
Na+ channel Nav1.9: in search of a gating mechanism.
Trends Neurosci.,
2003
Feb
, 26 (55-7).
Dib-Hajj S
et al.
NaN/Nav1.9: a sodium channel with unique properties.
Trends Neurosci.,
2002
May
, 25 (253-9).
Maingret F
et al.
Inflammatory mediators increase Nav1.9 current and excitability in nociceptors through a coincident detection mechanism.
J. Gen. Physiol.,
2008
Mar
, 131 (211-25).
Fjell J
et al.
Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons.
Brain Res. Mol. Brain Res.,
1999
Apr
20
, 67 (267-82).
Waxman SG
et al.
Nav1.9, G-proteins, and nociceptors.
J. Physiol. (Lond.),
2008
Feb
15
, 586 (917-8).
Zhang J
et al.
N-type fast inactivation of a eukaryotic voltage-gated sodium channel.
Nat Commun, 20220517, 13 (2713).
Dib-Hajj SD
et al.
NaV1.9: a sodium channel linked to human pain.
Nat. Rev. Neurosci.,
2015
Sep
, 16 (511-9).
Benn SC
et al.
Developmental expression of the TTX-resistant voltage-gated sodium channels Nav1.8 (SNS) and Nav1.9 (SNS2) in primary sensory neurons.
J. Neurosci.,
2001
Aug
15
, 21 (6077-85).
Liu CJ
et al.
Direct interaction with contactin targets voltage-gated sodium channel Na(v)1.9/NaN to the cell membrane.
J. Biol. Chem.,
2001
Dec
7
, 276 (46553-61).
Lopez-Santiago LF
et al.
Na+ channel Scn1b gene regulates dorsal root ganglion nociceptor excitability in vivo.
J. Biol. Chem.,
2011
Jul
1
, 286 (22913-23).
Lin Z
et al.
Biophysical and Pharmacological Characterization of Nav1.9 Voltage Dependent Sodium Channels Stably Expressed in HEK-293 Cells.
PLoS One, 2016, 11 (e0161450).
Laedermann CJ
et al.
Post-translational modifications of voltage-gated sodium channels in chronic pain syndromes.
Front Pharmacol, 2015, 6 (263).
Tyrrell L
et al.
Glycosylation alters steady-state inactivation of sodium channel Nav1.9/NaN in dorsal root ganglion neurons and is developmentally regulated.
J. Neurosci.,
2001
Dec
15
, 21 (9629-37).
Bao L
Trafficking regulates the subcellular distribution of voltage-gated sodium channels in primary sensory neurons.
Mol Pain,
2015
, 11 (61).
Sizova DV
et al.
A 49-residue sequence motif in the C terminus of Nav1.9 regulates trafficking of the channel to the plasma membrane.
J Biol Chem, 20200124, 295 (1077-1090).
Han C
et al.
The Domain II S4-S5 Linker in Nav1.9: A Missense Mutation Enhances Activation, Impairs Fast Inactivation, and Produces Human Painful Neuropathy.
Neuromolecular Med.,
2015
Jun
, 17 (158-69).
Huang J
et al.
A Novel Gain-of-Function Nav1.9 Mutation in a Child With Episodic Pain.
Front Neurosci, 2019, 13 (918).
Leipold E
et al.
A de novo gain-of-function mutation in SCN11A causes loss of pain perception.
Nat. Genet.,
2013
Sep
15
, ().
Schrenk-Siemens K
et al.
Translational Model Systems for Complex Sodium Channel Pathophysiology in Pain.
Handb Exp Pharmacol, 2018, 246 (355-369).
Hockley JR
et al.
The voltage-gated sodium channel NaV 1.9 in visceral pain.
Neurogastroenterol Motil, 2016Mar, 28 (316-26).
Kokotović T
et al.
Transcription factor mesenchyme homeobox protein 2 (MEOX2) modulates nociceptor function.
FEBS J, 2022Jun, 289 (3457-3476).
Li CL
et al.
N58A Exerts Analgesic Effect on Trigeminal Neuralgia by Regulating the MAPK Pathway and Tetrodotoxin-Resistant Sodium Channel.
Toxins (Basel), 20210517, 13 ().
Credits
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/128/ , accessed on 2024 May 08
