Kv2.1
Description: potassium voltage gated channel, Shab-related subfamily, member 1 Gene: Kcnb1 Alias: Kv2.1, kcnb1, kcr1-1, drk1pc, shab
Kv2.1, encoded by the gene KCNB1, is a member of the potassium voltage-gated channel subfamily B. Kv2.1 is a predominant delayed rectifier K+ current that regulates neuronal excitability, action potential duration and tonic spiking.
Experimental data
Rat Kv2.1 gene in CHO host cells datasheet |
||
|
Click for details 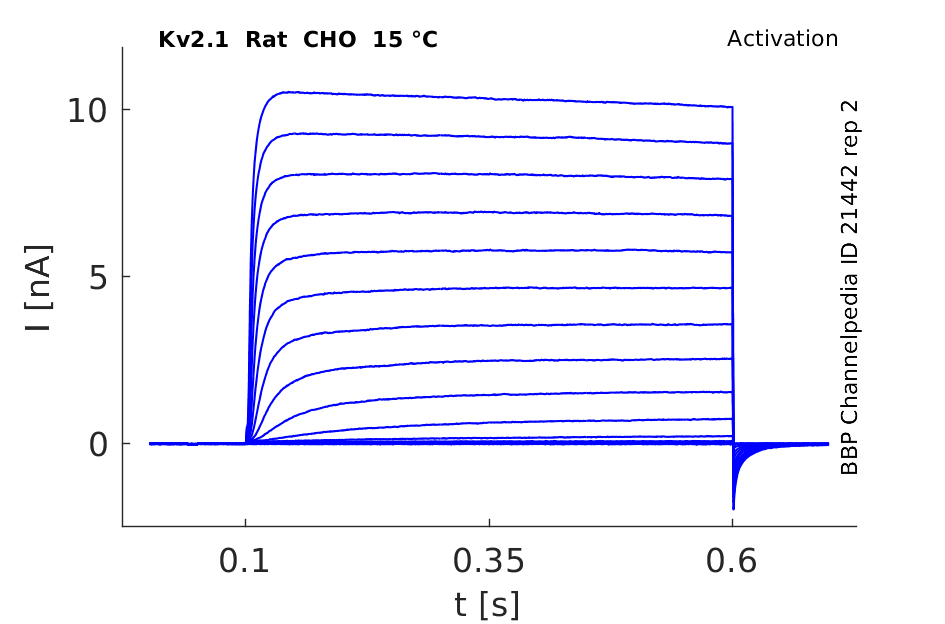
15 °Cshow 109 cells |
Click for details 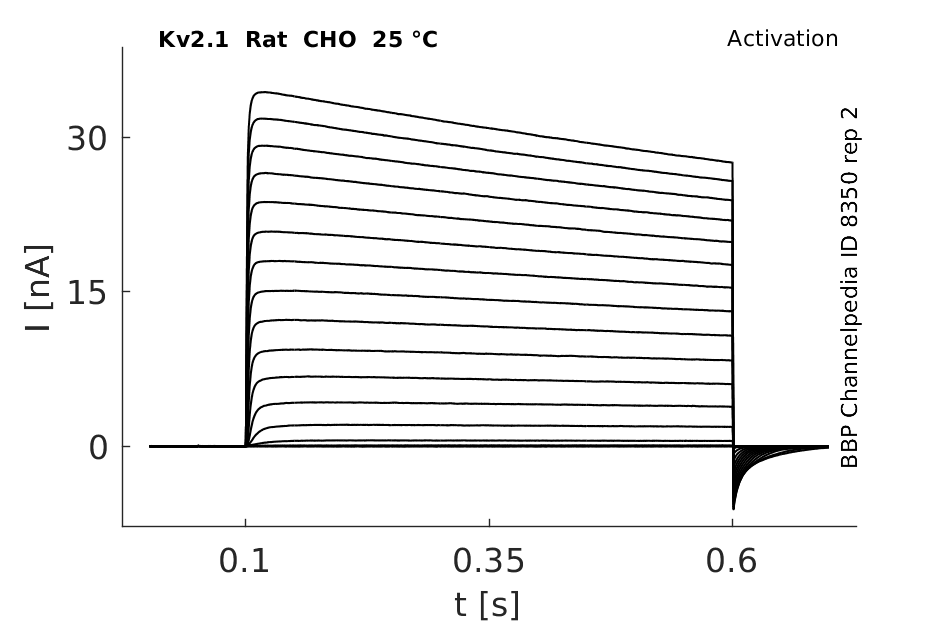
25 °Cshow 114 cells |
Click for details 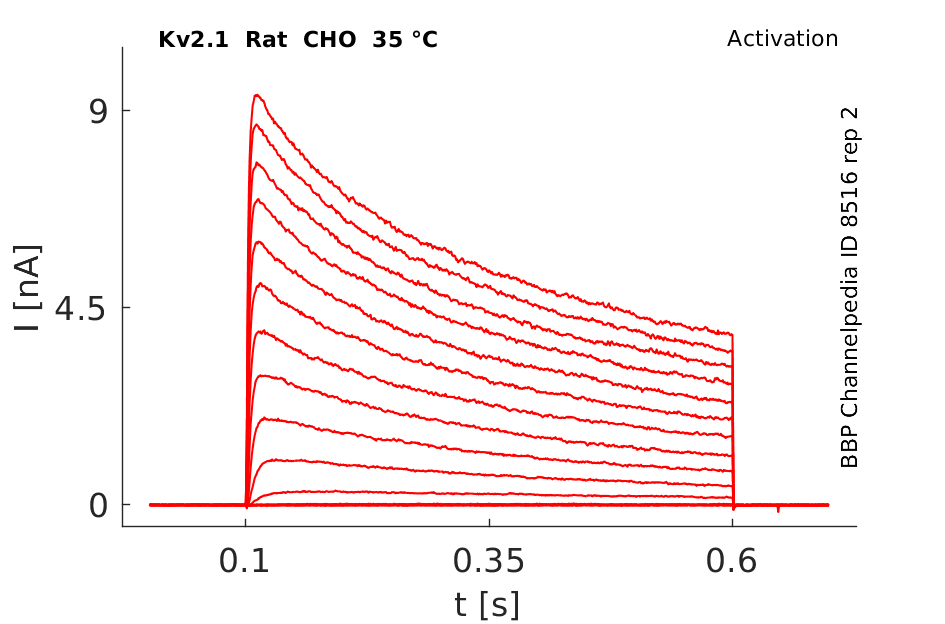
35 °Cshow 109 cells |
Mouse Kv2.1 gene in CHO host cells datasheet |
||
|
Click for details 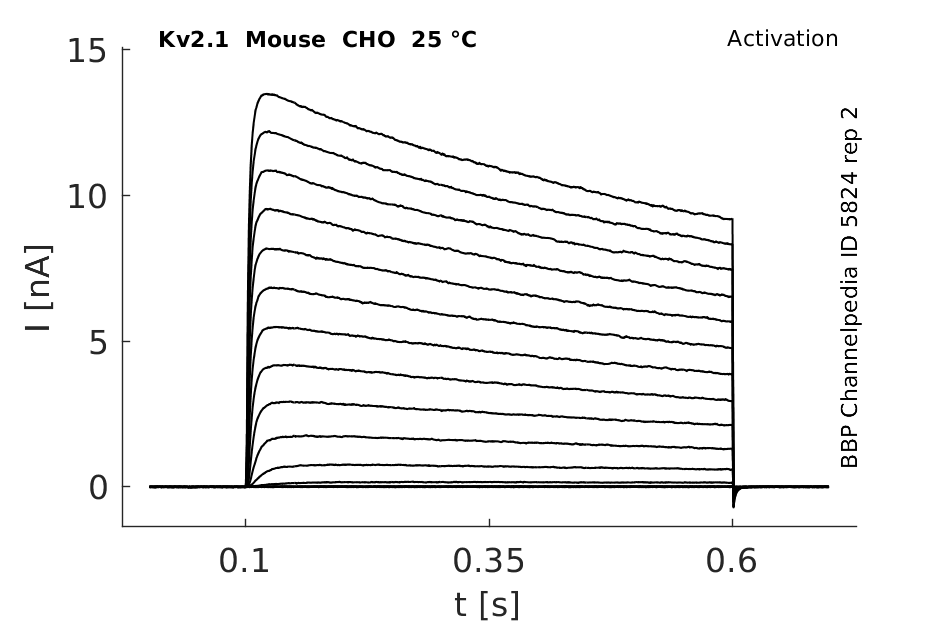
25 °Cshow 64 cells |
Click for details 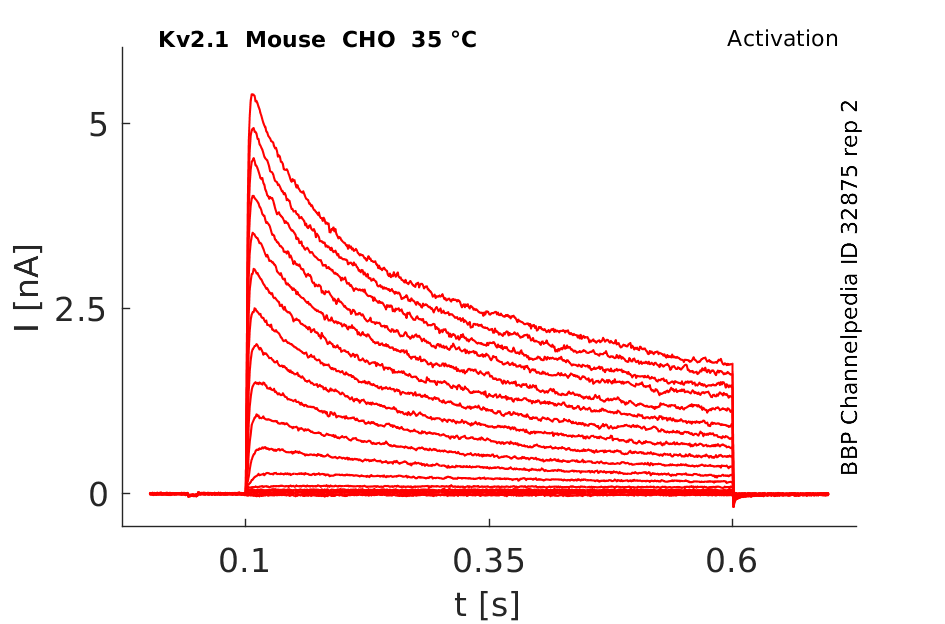
35 °Cshow 81 cells |
|
Human Kv2.1 gene in CHO host cells datasheet |
||
|
Click for details 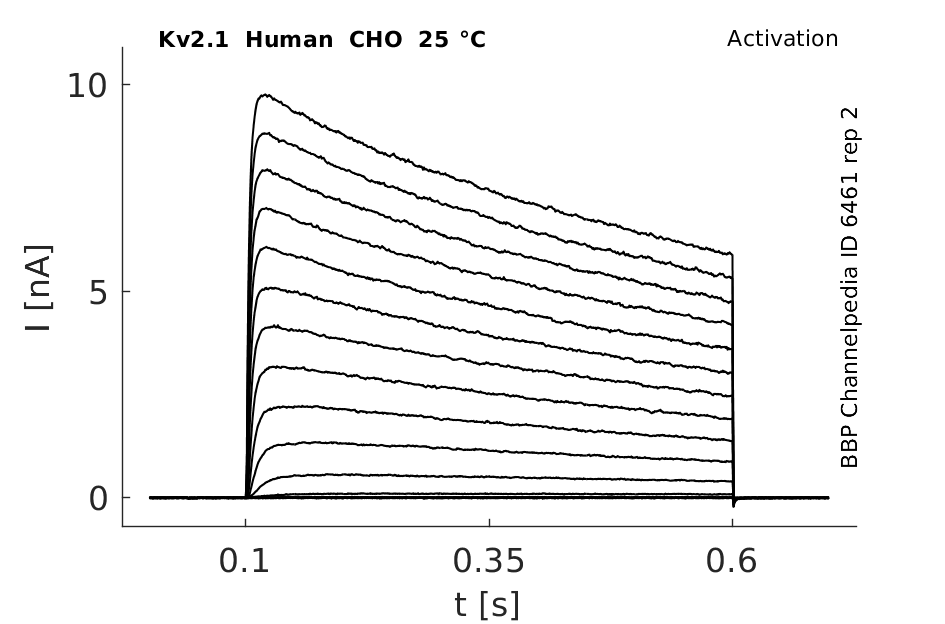
25 °Cshow 68 cells |
Click for details 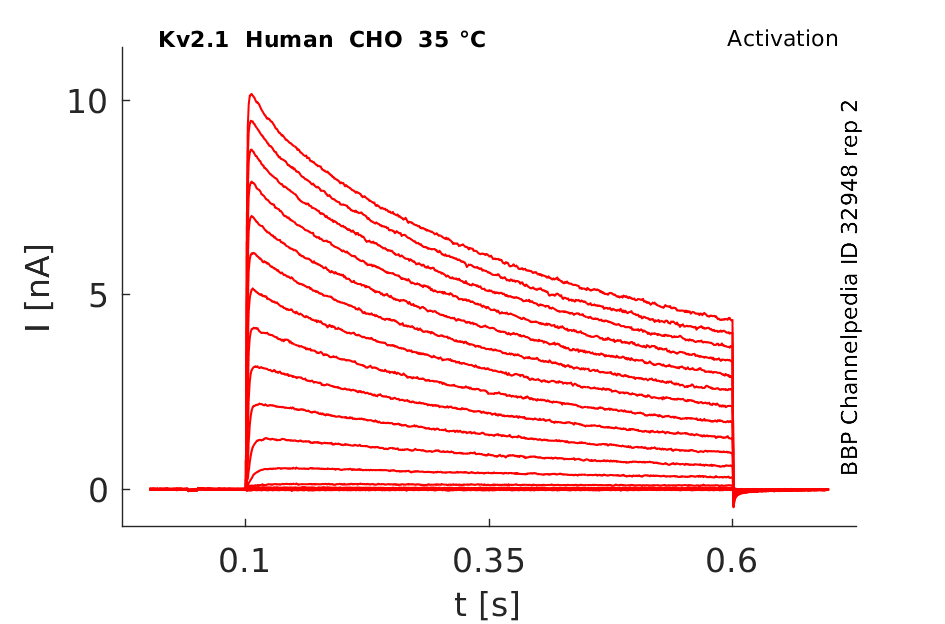
35 °Cshow 59 cells |
|
Rat Kv2.1 gene in HEK host cells datasheet |
||
|
Click for details 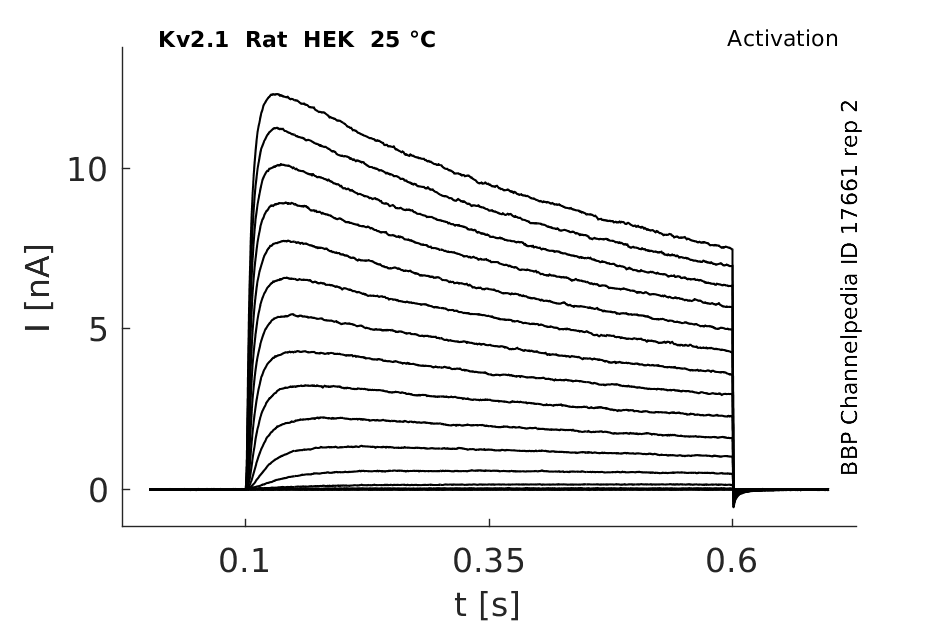
25 °Cshow 150 cells |
Click for details 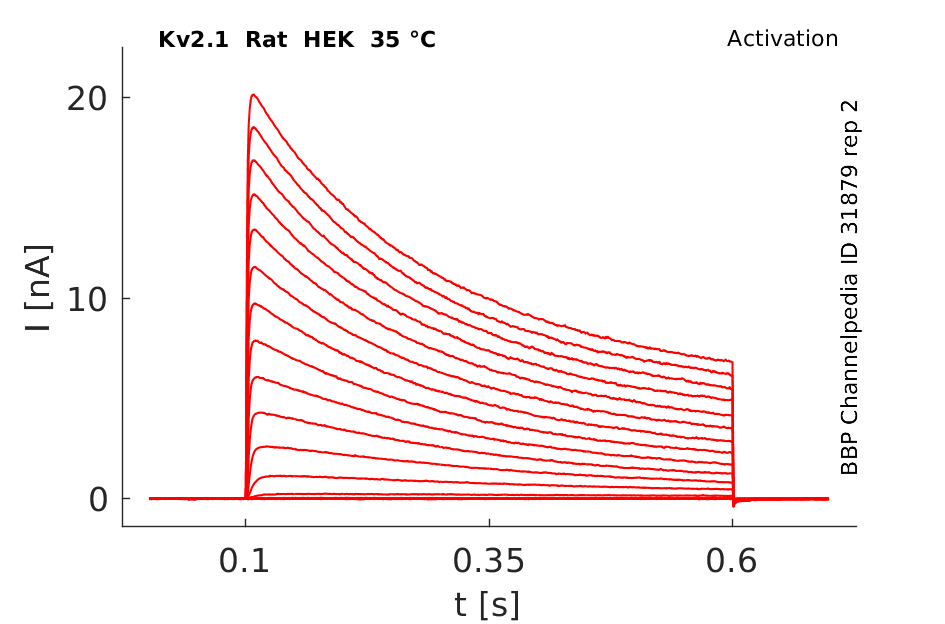
35 °Cshow 43 cells |
|
Rat Kv2.1 gene in CV1 host cells datasheet |
||
|
Click for details 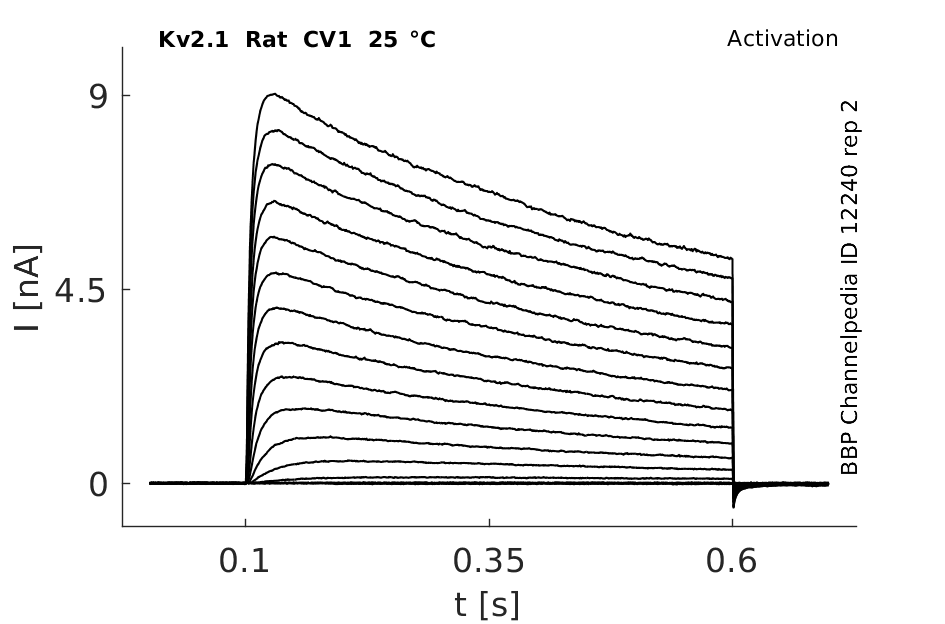
25 °Cshow 104 cells |
Click for details 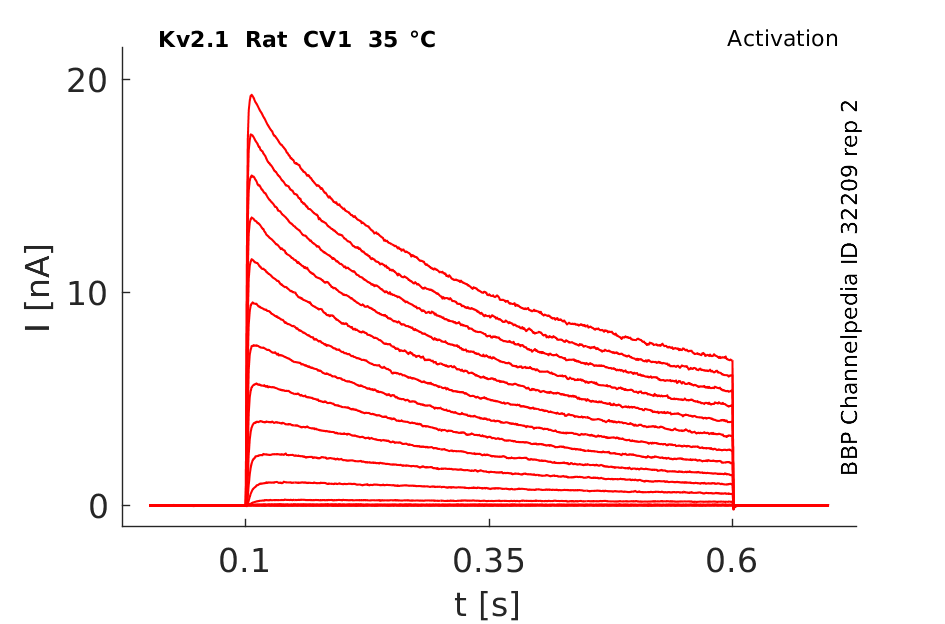
35 °Cshow 85 cells |
|
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_004975.4 | 11871 | |
| Mouse | NM_008420.4 | 11153 | |
| Rat | NM_013186.2 | 11086 |
The Kv2.1 K+ channel α-subunit polypeptide has the longest cytoplasmic C-terminal domain of any Kv channel at 440 amino acids. The vast majority of this domain is unique to Kv2.1; thus antibodies raised against sequences within this domain would be expected to recognize Kv2.1 selectively [732]
Isoforms
Phosphorylation
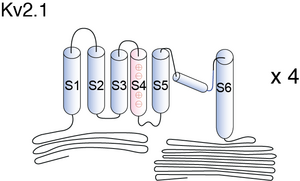
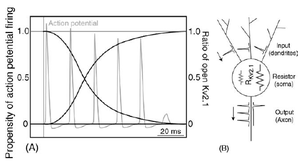
Dynamic calcineurin-dependent dephosphorylation of neuronal Kv2.1 channels in response to increased excitatory activity, epileptic seizures, hypoxia/ischaemia, and neuromodulatory stimuli leads to graded enhancement of Kv2.1 activity by lowering the voltage-dependent activation threshold and accelerating channel activation kinetics. These graded changes in activation allow Kv2.1 to homoeostatically suppress neuronal excitability, especially during periods of high-frequency action potential firing, and play a neuroprotective role under diverse hyperexcitable conditions. Kv2.1 channels consist of four ?-subunit polypeptides, each composed of six membrane-spanning segments (S1–S6) and large cytoplasmic N- and C-termini. The membrane spanning S1-S6 domains comprise approx 25% of the polypeptide, and upon tetrameric assembly form the voltage sensing andK+ ion-selective pore components of the channel. Almost 75% of Kv2.1 protein is cytoplasmic, with the cytoplasmic C-terminus comprising over 50% of the Kv2.1-subunit. The large intracellular regions can mediate interactions with diverse cellular components, and can be targeted by cellular protein kinases and phosphatases to achieve reversible covalent modification of channel structure and function.[62]
Visual Representation of Kv2.1 Structure
Methodology for visual representation of structure available here
Basic Structure
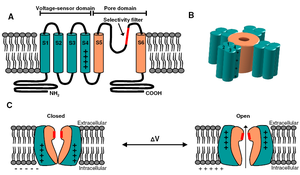
Individual Kv channel Kv2.1 alpha subunit polypepties have six transmembrane segments(termed S1-S6) and assemle post-translationlly to form tetrameric complexes . The ~300 amino acid core domains containing the transmembrane S1-S6 segments present in each of the four a subunits co-assemble to form the major portions of both the voltage-sensing apparatus and ion-selective pore. Amino- and carboxyl-termini are cytoplasmic, such that all extracellular domains are found within the core domain. The cytoplasmic N-terminus of Kv2.1 a subunits contains the tetramerization (Tl) domain that comprises the molecular determinant for subfamily (i.e. Kv2)-specific assembly of a subunits into functional tetrameric channels. Different Kv channel a subunits display a high degree of amino acid sequence similarity within the core domain and the Tl domain, but are more divergent in the carboxyl and non-Tl amino terminal domains. These cytoplasmic domains are involved in extensive intersubunit interactions within the tetramer, and in protein–protein interactions involved in regulating channel trafficking,localization and function.
Crystallography of Kv2.1
Kv2.1 has a glutamine residue (glutamine 290 in the paddle chimaera) corresponding to the first S4 arginine in Shaker (R1) and an additional preceding arginine labelled R0. Positively charged amino acids K5 and R6 form ionized hydrogen bonds with the internal negative cluster, whereas R3 and R4 form ionized hydrogen bonds with the external negative cluster. R0, R1 and R2 are positioned to interact favourably with the lipid phosphodiester layer, a mixed lipid–water environment and the water environment of the external aqueous cleft, respectively [1539]
Chimaera

Kv2.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Single channel conductance

Kv2.1 has a single channel conductance of ~10 pS when expressed in Xenopus
oocytes (Chapman et al., 2001; Pascual et al., 1997;Taglialatela and Stefani, 1993). The activation and inactivation time constants are 10 ms (at 0 mV)
and 3-5 s (at 10 mV in Xenopus oocytes), respectively (VanDongen et al., 1990).
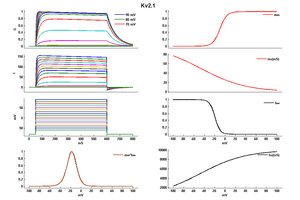
EFFECT OF TEMP ON Kv2.1 KINETICS

Rat/Human Kv2.1 channel
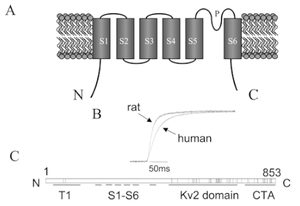
Voltage gated rearrangement of C and N termini
by using FRET combined with patch clamp technique, we have shown that there are voltagegated rearrangements between the N and C termini of the Kv2.1 channel. These movements are in the plane of the membrane and may (or may not) occur in a concerted manner between the four subunits. From this very rough approximation we speculate that the voltagegated movements of the Kv2.1-labeled termini (R(+60)-R(-80)) occur in a linear range of at least 1-2 nm [1541]
Exploring the voltage gated movement between the termini
To characterize electrophysiological properties of the labeled channels, we recorded K+ currents from COS1 cells of approximately similar size transfected by Kv2.1-ECFPC, Kv2.1-EYFPC, EYFPN-Kv2.1, and EYFPN-Kv2.1-ECFPC. Representative traces of the K+ current evoked by step depolarizations from a Vh of -80 mV and the corresponding currentvoltage (I-V) relationships are shown below. All four fluorescent channels showed slowly activating outward K+ currents that exhibited little or no inactivation during 200-ms depolarizations typical for the wild-type Kv2.1. The time constant of activation with step to +60 mV was 5.8 ± 0.8 ms (n = 8), 6.7 ± 3.0 ms (n = 3), 4.0 ± 0.7 ms (n = 8), and 5.4 ± 1.4 ms (n = 6) for the Kv2.1-EYFPC, Kv2.1-ECFPC, EYFPN-Kv2.1, and EYFPN-Kv2.1-ECFPC channels, respectively [1541]
Regulation of Kv2.1 conductance by cell surface channel density
In transfected HEK cells, Kv2.1 channels within cluster microdomains are nonconducting. Using total internal reflection fluorescence microscopy, the number of GFP-tagged Kv2.1 channels on the HEK cell surface was compared with K(+) channel conductance measured by whole-cell voltage clamp of the same cell. This approach indicated that, as channel density increases, nonclustered channels cease conducting. At the highest density observed, only 4% of all channels were conducting. Endogenous Kv2.1 levels were compared with the number of conducting channels determined by whole-cell voltage clamp. Only 13 and 27% of the endogenous Kv2.1 was conducting in neurons cultured for 14 and 20 d, respectively. Together, these data indicate that the nonconducting state depends primarily on surface density as opposed to cluster location and that this nonconducting state also exists for native Kv2.1 found in cultured hippocampal neurons [1545]
Measurement of GFP-Kv2.1 channel cell surface density using single-channel fluorescence

Kv2.1 Inactivation
The voltage dependence of inactivation was U-shaped, with maximum inactivation near 0 mV. During a maintained depolarization, development of inactivation was slow and only weakly voltage dependent (tau = 4 s at 0 mV; tau = 7 s at +80 mV). However, recovery from inactivation was strongly voltage dependent (e-fold for 20 mV) and could be rapid (tau = 0.27 s at -140 mV). Kv2.1 showed cumulative inactivation, where inactivation built up during a train of brief depolarizations. A single maintained depolarization produced more steady-state inactivation than a train of pulses, but there could actually be more inactivation with the repeated pulses during the first few seconds. We term this phenomenon "excessive cumulative inactivation." These results can be explained by an allosteric model, in which inactivation is favored by activation of voltage sensors, but the open state of the channel is resistant to inactivation.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1299522/
Kv2.1 Expressed in CHO

Human Kv2.1 expressed in COS-7 cells
In COS-7 cells, transiently transfected with human Kv2.1 plasmids, the effects of 17b-estradiol on whole-cell currents were recorded. The macroscopic currents from Kv2.1 were elicited by voltage steps from –80 to +60 Mv and substantially inhibited by application of 500 nM 17 beta-estradiol. The I–V relationship displayed markedly the inhibitory effects. At a potential of +60 mV, Kv2.1 currents were reduced by 42 % compared to control.[2049] Dominant expression of Kv2.1 in osteoblast-like MG63 cells may contribute to similar outward voltage-gated K currents and inhibition by 17 beta-estradiol in this cell type too.[2049]
U-type inactivation
Kv2.1 exhibits a distinct U-shaped voltage-dependend inactivation and closed-state inactivation (U-type inactivation). A mutation study of Kv2.1 (Y380) showed that C-type inactivation, occurring in Shaker channels, and U-type inactivation of Kv2.1 channels rely on dictinct molecular mechanisms.[2050]
De novo missense mutation in KCNB1 affects ion selectivity
De novo missense mutation in KCNB1, associated with epileptic encephalopathies, may cause the loss of ion selectivity. KV2.1-WT channels expressed in CHO cells displayed large voltage-dependent potassium currents with outward rectification and late inactivation. However, the expression of each of the three mutants led to small currents with linear I-V relationships. [2051]
(±)3,4-methylenedioxyamphetamine (MDA)
MDA was observed to decreased the delayed outward current of tetraethylammonium-sensitive cells in the hippocampus of neonatal rats. It was also shown to inhibited the K+ current in Kv2.1-expressing lung epithelial H1355 cells.[2052]
Kv2.1 currents and inhibition by paclitaxel in H9c2 cells

Kv2.1 and Kv2.1 V378A mutant recorded in CHO-K1 cells
Currents from Kv2.1- or Kv2.1 V378A mutant-expressing CHO-K1 cells were recorded by whole-cell patch-clamp technique. The results indicated that the mutation in KCNB1 associated with epileptic encephalopathy disrupts ion selectivity of Kv2.1 V378A. The V378A variant of the alpha subunit acts therefore as a non-selective cation channel that could be plausiby linked to the human disease phenotype.[2058]
Kv2.1 channels in HEK293 cells and effect of perifosine
Whole cell Kv2.1 currents were mesured in HEK293 cells in the absence and presence of the alkylphospolipid perifosine. The application of perifosine was shown to decrease Kv2.1 currents independently from concentration. Perifosine did not alter the voltage dependence of channel activation, but induced a hyperpolarizing shift in the voltage dependence of Kv2.1 channels inactivation. Thus the decrease of the current amplitude may be due to the modification of Kv2.1 inactivation gating by perifosine.[2060]
An allosteric model for inactivation of Kv2.1
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1299522/
Model Kv2.1 (ID=23)
| Animal | Xenopus | |
| CellType | oocyte | |
| Age | 0 Days | |
| Temperature | 23.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [277] A M VanDongen et. al; Neuron 1990 Oct | |
| mpower | 1.0 | |
| m Inf | 1/(1+exp(((v -(-9.200))/(-6.600)))) | |
| m Tau | 100.000/(1+exp(((v -(-46.560))/(44.140)))) | |
| hpower | 1.0 | |
| h Inf | 1/(1+exp(((v -(-19.000))/(5.000)))) | |
| h Tau | 10000.000/(1+exp(((v -(-46.560))/(-44.140)))) | |
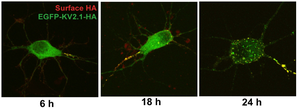
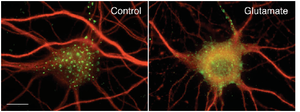
The idea that these changes in Kv2.1 localization occur as a result of activity-dependent Kv2.1 dephosphorylation is supported by their similar activity dependence in vivo and in culture. Moreover, in cultured neurons the changes in Kv2.1 localization and phosphorylation state exhibit similar glutamate dose responses, kinetics and pharmacology. Notably, the Kv2.1 clustering signal is located in a serine- and threonine-rich cytoplasmic tail of Kv2.1, and the clustering signal itself contains three serine residues that disrupt clustering when individually mutated to alanine.[174]
Exogenously expressed Kv2.1 targets to the axon initial segment in cultured hippocampal neurons before accumulating on the soma.[299]
Cyclin E1
Cyclin E1 was shown to be involved in regulating the phosphorylation status and localization of Kv2.1 channels. The overexpression of cyclin E1 in cortical neurons of rats was seen to disperse Kv2.1 channel clusters.[1542]
Cell cycle-dependent regulation of Kv2.1
Cell cycle-dependent changes in localization and phosphorylation of Kv2.1 impact the contact sites endoplasmic reticulum membrane in COS-1 and CHO cells. However, this cell cycle-dependent phosphorylation of Kv2.1 was not observed to change the electrophysiological properties of CHO cells.[2056]
Redistribution of Kv2.1 clusters after nerve lesion
Immunohistochemistry revealed that lesion of peripheral axons alters the membrane distribution of Kv2.1 channels in motoneurons. While reinnervation did not fully rehabilitate Kv2.1 channel distribution, Kv2.1 cluster sizes were observed to fully recover in the absence of reinnervation. These results suggest that Kv2.1 redistribution after nerve injury may to a great extent be independent of reinnervation.[2070]
Kv2.1 Localization in Hippocampal Neurons
The Kv2.1 voltage-gated K(+) channel is found both freely diffusing over the plasma membrane and concentrated in micron-sized clusters localized to the soma, proximal dendrites, and axon initial segment of hippocampal neurons [1545]
Kv2.1 is localized uniquely among mammalian brain K+channels to large clusters on the soma and on the very proximal portions of dendrites [732]
Kv2.1 channels are found in clusters on the soma and proximal dendrites [1623]

The voltage gated delayed rectifier type K+ channel kv2.1 is expressed in high density clusters in the on the soma and proximal dendrites of mammalian central neurons. Thus dynamic regulation of Kv2.1 would be predicted to have an effect on neuronal excitability [1826]
Kv2.1 clusters on the AIS
Voltage-gated ion channels are densly expressed at the membrane of the axon initial segment (AIS) pivotal for the initiation of action potentials. Kv2.1 clusters on the AIS represent specialized domains harbouring various signaling pathways. These sites are deficient in Ankyrin-G across various mammalian species. Differences in Kv2.1 phosphorylation likely modulate membrane exitability in this axonal compartment.[2071]
Distribution of Kv2.1 subunits in different neuronal compartments
Approximately the same densities of Kv2.1 are expressed in somatic, proximal dendritic and AIS membranes. The distribution of Kv2.1 across the cell surface was found to be non-uniform. Kv2.1 clusters are often near GABAergic synapses but do not overlap with them.[2072]
Striatal medium spiny neurons differ in Kv2.1 cluster expression
In striatal medium spiny neurons (MSNs) Kv2.1 clusters were shown by electron microscopy-immunogold labelin to be juxtaposed to clusters of intracellular ryanodine receptor (RyR) Ca2+-release channels and adjacent to subsurface cisternae.[2076]
Induction of ER-plasma-membrane junctions by Kv2.1
Experiments conducted in transfected HEK 293 cells and cultured hippocampal neurons demonstrated that clustered Kv2.1 channels induce stable ER-plasma-membrane junctions. The application of glutamate was found to reverse Kv2.1 clustering and retraction of the ER frome the cell membrane.[2080]
Kv2.1 facilitation of exocytosis by interaction with syntaxin

Role in neuronal signaling
Kv2.1 deficiency leads to neuronal and behavioural hyperexcitability
Field recordings from hippocampal slices of Kv2.1-/- mice reveal hyperexcitability in response to the convulsant bicuculline, and epileptiform activity in response to stimulation. In Kv2.1-/- mice, long-term potentiation at the Schaffer collateral - CA1 synapse is decreased. Kv2.1-/- mice are strikingly hyperactive, and exhibit defects in spatial learning, failing to improve performance in a Morris Water Maze task. Kv2.1-/- mice are hypersensitive to the effects of the convulsants flurothyl and pilocarpine, consistent with a role for Kv2.1 as a conditional suppressor of neuronal activity. Although not prone to spontaneous seizures, Kv2.1-/- mice exhibit accelerated seizure progression. Together, these findings suggest homeostatic suppression of elevated neuronal activity by Kv2.1 plays a central role in regulating neuronal network function [1535]
Bladder Overactivity
mRNA level of Kv2.1 decreased significantly in rat bladder with DH, which was one of the important pathogenetic mechanisms for DH (detrusor hyperreflexia), and suggested that Kv2.1 might be one of the therapeutic targets for bladder overactivity [1747]
Channel Gating
Neurotransmitters and neuronal stress trigger dephosphorylation of Kv2.1 in a calcineurin-dependent manner, which disperses Kv2.1 clusters and changes the channel-gating properties. A novel proximal restriction and clustering sequence (PRC) in the C-terminus of Kv2.1 seems to be necessary and sufficient for clustering. Because these channels open and close slowly, they reduce repetitive spiking and could contribute to homeostatic plasticity [1623]
Pulmonary Hypertension
Cigarette smoke exposure may be involved in pulmonary hypertension by downregulating potassium channels Kv1.5 and Kv2.1 mRNA expression in rat pulmonary artery smooth muscles.
Kv2.1 in CHO cells regulated by phosphorylation
A comparative study of wild type and mutant variants of Kv2.1 channels expressed in CHO cells revealed the importance of intracellular N-terminal domains in regulating the phosphorylation of the C-terminal of Kv2.1. Proaptotic membrane insertion necessitates these regulatory events.[2054]
C-terminus regulates Kv2.1 localization but not conductive properties
A mutation study using cultured pyramidal neurons and HEK293 cells compared the localization and electrophysiological properties of the cells expressing either WT Kv2.1 channels or Kv2.1 channels carrying an altered C-terminus. The results showed that the C-terminus mutation removed Kv2.1 from cell-surface clusters but that the steady-state voltage-dependence was not modulated by this domaine.[2063]
Kv2.1 regulation of insulin secretion in β-cells
Kv2.1 -/- mice diplay reduced K+ currents and enhanced insulin secretion in pancreatic β-cells, Kv2.2 silencing, however, increased the release of somatostatin from pancreatic islet δ-cells.[1735]
KCNB1 mutation associated to type 2 diabetes
A linkage between an SNP of KCNB1 (rs1051295) and type 2 diabetes was suggested from a study comparing beta cell activity and insulin sensitivity among patients charing the SNP and a control group.[2064]
Kv2 channels regulate firing rate in pyramidal neurons
Whole-cell voltage-clamp recordings of cultered neurons transfected with a Kv2,1 pore mutant had a decreased outward current and the total current density was reduced by ~ 45 %. These results indicate that Kv2.1 channels control the membrane potential during the interspike interval regulating the firing rate.[2066]
Kv2 downregulation after nerve lesion
Axonal injury-induced downregulation of Kv2.1 and Kv2.2 channels contributes to fidelity of repetitive firing during prolonged input. Physiological role of Kv2 channels may therefore consist in limiting neuronal excitability.[2047]
Kv2.1 dephosphorylation in synaptic plasticity
Increases in synaptic activity were shown to result in the dephosphorylation of Kv2.1 subunits. The dephosphorylation of Kv2.1 channels via protein phosphatase-1 (PP1) was thus suggested to contribute to NMDA receptor-induced synaptic plasticity.[2077]
Immunorepressive effect of Kv2.1 inhibition in T cells###
A immunorepression study highlighted the distribution of Kv2.1 channels in CD4(+) and CD8(+) cells which express the Kv2.1 channels mainly extracellularly. In vivo evidence points at the protective effect of Kv2.1channel-blocking scorpion toxins Ts6 and Ts15 on autoimmune hypersensitivity.[2078]
KCNB1 Polymorphisms associated with metabolic disorders###
An gene association study linked polymorphisms of KCNB1 with the resistance or prediposition to the pertubation of lipid metabolism and insulin sensitivity.[2079]
Leptin modulates repolarisation via Kv2.1 surface expression
In pancreatic pancreatic β-cells Kv2.1 channels are the dominant delayed rectifier potassium channels facilitating AP repolarization. Leptin was shown to increase the surface expression of Kv2.1 channels leading to a more rapid repolarization of the membrane during action potentials. Leptin regulates Kv2.1 channels as part of a concerted inhibition of insulin secretion.[2082]
KCNB1 mutation cause deficient firing and infantile epilepsy
Mutations of the K+ channel-coding gene KCNB1 in the voltage sensor(p.R306C) were observed to disrupt sensor sensitivity and in the pore domain (p.G401R) abolished Kv2 currents in transfected pyramidal neurons. Both mutations disrupted repetitive neuronal firing. KCNB1 mutations can be causal for infantile epilepsy, deficient firing of pyramidal neurons and perturbation of neuronal circuit stability.[2084]
Kv6.3, Kv10.1, Kv11.1
HaTx
Gating modifier peptides bind to ion channels and alter the gating process of these molecules. One of the most extensively studied peptides Hanatoxin(HaTx), isolated from a Chiliean tarantula, has been used to characterize the blocking properties of the voltage gated potassium channel Kv2.1. Advanced simulation results support the concept of mutual conformational changes upon peptide binding to the S3b region of the channel which will restrict movement of S4 and compromise coupling of the gating machinery to opening of the pore.[61]
Jingzhaotoxin
A blocker Specific to ONLY Kv2.1, often used to eradicate the effects of kv2.1 subunits for isolation of other ion channels [1657]
Syntaxin-1A
Earlier works on Syn-1A-Ca2+ channel interaction, taken together with our works examining how Syn-1A inhibits Kv2.1 and KATP channels, strongly suggest that Syn-1A changes its conformation not only to prepare for and promote vesicle exocytotic fusion, but also to modulate the gating of these ion channels in a manner that optimally regulates membrane excitability. Thus, during priming for exocytosis in Beta-cells, open form Syn-1A promotes KATP channel closing.and the ensuing membrane depolarization. Once Beta-cells are depolarized, open form Syn-1A may prolong depolarization by strongly inhibiting Kv2.1 (therefore K+ efflux). This action, together with the reduced inhibition of VDCC by open form Syn-1A, would collectively optimize Ca2+ influx during exocytosis. Direct regulation of ion channels by other exocytotic proteins is much less known, although RIM and GEFII have been implicated to interact with VDCC and KATP channels, respectively. Our recent report showing that Munc13-1 intimately interacts with GEFII and RIM to modulate insulin exocytosis may suggest that this complex could also somehow modulate VDCC and KATP channels as part of the priming process for secretion.Alternatively, Munc13-1 and tomosyn may modulate VDCC and K+ channels (Kv and KATP) via their ability to alter the conformation of Syn-1A.[63]
GDF-15 regulates Kv2.1-mediated outward K+ current
The GDF-15 (member of the transforming growth factor-β (TGF-β)-induced amplification of IK is mediated by the increased expression and reduced lysosome-dependent degradation of the Kv2.1 protein, the main α-subunit of IK. Exposure of CGNs to GDF-15 markedly induced the phosphorylation of ERK, Akt, and mTOR, but the GDF-15-induced IK densities and increased expression of Kv2.1 were attenuated only by Akt and mTOR inhibitors, not ERK inhibitors. Pharmacological inhibition of the Src-mediated phosphorylation of TβRII, not TβRI, abrogated the effect of GDF-15 on IK amplification and Kv2.1 induction [1534]
pGSN protects HIV-1 gp120-Induced neuronal injury via voltage-gated K+ channel Kv2.1
Treatment with pGSN (a secreted form of gelsolin) suppressed the gp120-induced increase of delayed rectifier current (IK) and reduced vulnerability to gp120-induced neuronal apoptosis. Application of Guangxitoxin-1E (GxTx), a Kv2.1 specific channel inhibitor, inhibited gp120 enhancement of IK and associated neuronal apoptosis, similar effects to pGSN. Taken together, these results indicate pGSN protects neurons by suppressing gp120 enhancement of IK through Kv2.1 channels and reduction of pGSN in HIV-1-infected brain may contribute to HIV-1-associated neuropathy. [1536]
Cyclin E1 regulates Kv2.1
Recently, cyclin-dependent kinase 5 (Cdk5) was shown to phosphorylate Kv2.1, with pharmacological Cdk5 inhibition being sufficient to decluster channels. In another study, cyclin E1 was found to restrict neuronal Cdk5 kinase activity. We show here that cyclin E1 regulates Kv2.1 cellular localization via inhibition of Cdk5 activity. Expression of cyclin E1 in human embryonic kidney cells prevents Cdk5-mediated phosphorylation of Kv2.1, and cyclin E1 overexpression in rat cortical neurons triggers dispersal of Kv2.1 channel clusters [1542]
SsmTx-I, a Specific Kv2.1 blocker
By whole-cell recording, SsmTx-I significantly blocked voltage-gated K(+) channels in dorsal root ganglion neurons with an IC50 value of 200 nM, but it had no effect on voltage-gated Na(+) channels. Among the nine K(+) channel subtypes expressed in human embryonic kidney 293 cells, SsmTx-I selectively blocked the Kv2.1 current with an IC50 value of 41.7 nM, but it had little effect on currents mediated by other K(+) channel subtypes. Blockage of Kv2.1 by SsmTx-I was not associated with significant alteration of steady-state activation, suggesting that SsmTx-I might act as a simple inhibitor or channel blocker rather than a gating modifier.
SNARE Proteins
Physical interaction between the SNARE proteins, mainly syntaxin, and Kv channels (Kv1.1, Kv2.1) have been shown to modulate the gating function of the channels [64]
KC Antibody

Regulation of subunit Kv9.3
Voltage-gated potassium (Kv) channels containing alpha-subunits of the Kv2 subfamily mediate delayed rectifier currents in excitable cells. Channels formed by Kv2.1 alpha-subunits inactivate from open- and closed states with both forms of inactivation serving different physiological functions. Kv9.3 changes the state preference of Kv2.1 inactivation by accelerating closed-state inactivation and inhibiting open-state inactivation. An N-terminal regulatory domain (NRD) has been suggested to determine the function of the modulatory alpha-subunit Kv8.1. However, when we tested the NRD of Kv9.3, we found that the functional properties of chimeric Kv2.1 channels containing the NRD of Kv9.3 (Kv2.1(NRD)) did not resemble those of Kv2.1/Kv9.3 heteromers, thus questioning the role of the NRD in Kv9 subunits [1544]
Kv2.3

Kv2.1/Kv6.4/KCNE5 complex
Heteromeric complexes of Kv2.1, Kv6.4 and KCNE5 subunits can form in vivo. Currents in Kv2.1/Kv6.4-expressing HEK29 cells were efficiently modulated by the transmembrane β-subunit KCNE5. Assembly of such complexes may play a role in tissue-specific fine-tuning of cell excitability.[2055]
Curcumin modulates Kv2.1 in HEK293 cells
Currents of Kv2.1 channels expressed in HEK293 cells were significantly reduced by curcumin. The inhibition followed a slow time course and was partially reversible. However, under curcumin administration Kv2.1 channel voltage dependence of activation remained unaltered. Curcumin accelerated the kinetics of open- and closed-state inactivation and shifted hyperpolarization, indicating that curcumin affects the inactivation gating.[2057]
Kv2/KvS heterotetramers
Electrically silent KvS subunits (Kv5-Kv6 and Kv8-Kv9) form tissue-specific heterotetramers with Kv2 channels resulting in greater variability of Kv2 currents. Suppression of the Kv2.1/Kv6.4 closed-state inactivation by the channel blocker 4-aminopyridine lead to the recovery of Kv2.1/Kv6.4 channels inactivated a holding potential of -80 mV (resting potential). This indicates the modulation of the pharmacological responce of Kv2 channels when forming heterotetramers with KvS subunits.[2059]
Modulation of Kv2.1 by CCR5/CXCR4 in response to gp120
Exposure of hippocampal neurons to HIV-1 glycoprotein120 (gp120) elicits a neuroprotective Kv2.1-mediated response against non-apoptotic cell death. The modulation of Kv2.1 by the chemokine co-receptors CCR5 and CXCR4 induces neuroprotective changes via the phosphorylation and subsequent altered sub-cellular localization and activation properties of Kv2.1.[2065]
Treatment with plasma gelsolin (pGSN) diminished the HIV-1 gp120 induced augmentation of the Kv2.1-mediated delayed rectifier current and vulnerability to neuronal apoptosis.[1536]
Oxidative stress, syntaxin and apoptotic Kv2.1 currents
Oxidative stimuli elicit intracellular Ca(2+) release, activation of CaMKII, and Kv2.1 membrane insertion by interaction with syntaxin, promoting apoptotic K+ currents. The inhibition of CaMKII was observed to prevent the increased K(+) current and to enhance neuronal viability.[2067]
Leptin modulates in the voltage dependence of Kv2.1
The hypothalamic arcuate nucleus (ARH) harbours NPY neurons that display leptin-sensitive delayed rectifier K(+) currents. Leptin was observed to induce a hyperpolarizing shift in the voltage dependence of Kv2.1 channels. Therefore, leptin may modulate the intrinsic excitability of NPY neurons via Kv2.1 channels.[2069]
Subfamily-specific Kv2.1/Kv6.4 heterotetramerization involve N- and C-termini
FRET and co-IP approaches revealed that the Kv2.1 N-terminus interacts with the Kv6.4 C-terminus. Charge reversing substitutions on the N-terminal sequence CDD conserved between Kv2 and KvS proteins abolished the interaction between these subunits.[1841]
Cytoplasmic binding of syntaxin to Kv2.1
Binding of syntaxin to the cytoplasmic C-terminus of Kv2.1 was observed to be crucial for the increase of apoptotic K+ currents in oxidant-induced apoptosis.[2074]
Forskolin
Forskolin was shown to directly block Kv2.1 channels. In sympathetic neurons forskolin can reduce neuronal excitability partially via a Kv2.1 block.[2081]
NS5A prevents apoptotic K+ loss via inhibition of Kv2.1
The Hepatitis C virus protein NS5A prevents loss of intracellular potassium via Kv2.1 block impeding cell death. The phosphorylation status at CK2-directed residues was shown to enable NS5A1b-mediated inhibition of Kv2.1.[2083]
References
The Roles of N- and C-terminal determinants in the activation of the Kv2.1 potassium channel.
J. Biol. Chem.,
2003
Apr
11
, 278 (12769-78).
Wolf-Goldberg T
et al.
Target soluble N-ethylmaleimide-sensitive factor attachment protein receptors (t-SNAREs) differently regulate activation and inactivation gating of Kv2.2 and Kv2.1: Implications on pancreatic islet cell Kv channels.
Mol. Pharmacol.,
2006
Sep
, 70 (818-28).
McKay MC
et al.
Linoleic acid both enhances activation and blocks Kv1.5 and Kv2.1 channels by two separate mechanisms.
Am. J. Physiol., Cell Physiol.,
2001
Oct
, 281 (C1277-84).
Scholle A
et al.
Rate-limiting reactions determining different activation kinetics of Kv1.2 and Kv2.1 channels.
J. Membr. Biol.,
2004
Mar
15
, 198 (103-12).
Huang PT
et al.
The interaction of spider gating modifier peptides with voltage-gated potassium channels.
Toxicon,
2007
Feb
, 49 (285-92).
Mohapatra DP
et al.
Dynamic regulation of the voltage-gated Kv2.1 potassium channel by multisite phosphorylation.
Biochem. Soc. Trans.,
2007
Nov
, 35 (1064-8).
Leung YM
et al.
SNAREing voltage-gated K+ and ATP-sensitive K+ channels: tuning beta-cell excitability with syntaxin-1A and other exocytotic proteins.
Endocr. Rev.,
2007
Oct
, 28 (653-63).
Feinshreiber L
et al.
Voltage-gated potassium channel as a facilitator of exocytosis.
Ann. N. Y. Acad. Sci.,
2009
Jan
, 1152 (87-92).
Börjesson SI
et al.
Structure, function, and modification of the voltage sensor in voltage-gated ion channels.
Cell Biochem. Biophys.,
2008
, 52 (149-74).
Wray D
The roles of intracellular regions in the activation of voltage-dependent potassium channels.
Eur. Biophys. J.,
2004
May
, 33 (194-200).
Misonou H
et al.
Kv2.1: a voltage-gated k+ channel critical to dynamic control of neuronal excitability.
Neurotoxicology,
2005
Oct
, 26 (743-52).
Regulation of ion channel localization and phosphorylation by neuronal activity.
Nat. Neurosci., 2004 Jul , 7 (711-8).
Guan D
et al.
Kv2 subunits underlie slowly inactivating potassium current in rat neocortical pyramidal neurons.
J. Physiol. (Lond.),
2007
Jun
15
, 581 (941-60).
Zaks-Makhina E
et al.
Specific and slow inhibition of the kir2.1 K+ channel by gambogic acid.
J. Biol. Chem.,
2009
Jun
5
, 284 (15432-8).
VanDongen AM
et al.
Alteration and restoration of K+ channel function by deletions at the N- and C-termini.
Neuron,
1990
Oct
, 5 (433-43).
Sarmiere PD
et al.
The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ.
,
2008
, 9 (112).
Wood MJ
et al.
Two mechanisms of K(+)-dependent potentiation in Kv2.1 potassium channels.
Biophys. J.,
2000
Nov
, 79 (2535-46).
O'Connell KM
et al.
Localization-dependent activity of the Kv2.1 delayed-rectifier K+ channel.
Proc. Natl. Acad. Sci. U.S.A.,
2010
Jul
6
, 107 (12351-6).
Chung JJ
et al.
Biochemical characterization of the native Kv2.1 potassium channel.
FEBS J.,
2005
Jul
, 272 (3743-55).
Ottschytsch N
et al.
Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome.
Proc. Natl. Acad. Sci. U.S.A.,
2002
Jun
11
, 99 (7986-91).
Murakoshi H
et al.
Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons.
J. Neurosci.,
1999
Mar
1
, 19 (1728-35).
Veys K
et al.
Quantitative single-cell ion-channel gene expression profiling through an improved qRT-PCR technique combined with whole cell patch clamp.
J. Neurosci. Methods,
2012
Jul
30
, 209 (227-34).
Betancourt L
et al.
Potassium (K+) channel expression in basal forebrain cholinergic neurons.
J. Neurosci. Res.,
2000
Sep
15
, 61 (646-51).
Du J
et al.
The K+ channel, Kv2.1, is apposed to astrocytic processes and is associated with inhibitory postsynaptic membranes in hippocampal and cortical principal neurons and inhibitory interneurons.
Neuroscience,
1998
May
, 84 (37-48).
Mezey E
et al.
Transplanted bone marrow generates new neurons in human brains.
Proc. Natl. Acad. Sci. U.S.A.,
2003
Feb
4
, 100 (1364-9).
Wang CY
et al.
GDF-15 regulates Kv2.1-mediated outward K+ current through Akt/mTOR signaling pathway in rat cerebellar granule cells.
Biochem. J.,
2014
Mar
6
, ().
Speca DJ
et al.
Deletion of the Kv2.1 delayed rectifier potassium channel leads to neuronal and behavioral hyperexcitability.
Genes Brain Behav.,
2014
Feb
4
, ().
Liu H
et al.
Plasma gelsolin protects HIV-1 gp120-Induced neuronal injury via voltage-gated K(+) channel Kv2.1.
Mol. Cell. Neurosci.,
2013
Oct
24
, ().
Long SB
et al.
Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment.
Nature,
2007
Nov
15
, 450 (376-82).
Kobrinsky E
et al.
Molecular rearrangements of the Kv2.1 potassium channel termini associated with voltage gating.
J. Biol. Chem.,
2006
Jul
14
, 281 (19233-40).
Shah NH
et al.
Cyclin e1 regulates kv2.1 channel phosphorylation and localization in neuronal ischemia.
J. Neurosci.,
2014
Mar
19
, 34 (4326-31).
Chen M
et al.
Isolation and characterization of SsmTx-I, a Specific Kv2.1 blocker from the venom of the centipede Scolopendra Subspinipes Mutilans L. Koch.
J. Pept. Sci.,
2014
Mar
, 20 (159-64).
Kerschensteiner D
et al.
Structural determinants of the regulation of the voltage-gated potassium channel Kv2.1 by the modulatory alpha-subunit Kv9.3.
J. Biol. Chem.,
2003
May
16
, 278 (18154-61).
Fox PD
et al.
Regulation of Kv2.1 K(+) conductance by cell surface channel density.
J. Neurosci.,
2013
Jan
16
, 33 (1259-70).
Lai HC
et al.
The distribution and targeting of neuronal voltage-gated ion channels.
Nat. Rev. Neurosci.,
2006
Jul
, 7 (548-62).
Misonou H
Homeostatic regulation of neuronal excitability by k+ channels in normal and diseased brains.
Neuroscientist,
2010
Feb
, 16 (51-64).
Fowler PW
et al.
The pore of voltage-gated potassium ion channels is strained when closed.
Nat Commun,
2013
, 4 (1872).
Zhan XQ
et al.
The antidepressant citalopram inhibits delayed rectifier outward K⁺ current in mouse cortical neurons.
J. Neurosci. Res.,
2012
Jan
, 90 (324-36).
MacDonald PE
et al.
Temperature and redox state dependence of native Kv2.1 currents in rat pancreatic beta-cells.
J. Physiol. (Lond.),
2003
Feb
1
, 546 (647-53).
Li XN
et al.
The role of voltage-gated potassium channels Kv2.1 and Kv2.2 in the regulation of insulin and somatostatin release from pancreatic islets.
J. Pharmacol. Exp. Ther.,
2013
Feb
, 344 (407-16).
Gan XG
et al.
Expressions of voltage-gated K+ channel 2.1 and 2.2 in rat bladder with detrusor hyperreflexia.
Chin. Med. J.,
2008
Aug
20
, 121 (1574-7).
Chiara MD
et al.
A small domain in the N terminus of the regulatory alpha-subunit Kv2. 3 modulates Kv2.1 potassium channel gating.
J. Neurosci.,
1999
Aug
15
, 19 (6865-73).
Murakoshi H
et al.
Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation.
Mol. Pharmacol.,
1997
Nov
, 52 (821-8).
Lin CY
et al.
[Cigarette smoke exposure induced pulmonary artery pressure increase through inhibiting Kv1.5 and Kv2.1 mRNA expression in rat pulmonary artery smooth muscles].
Zhonghua Jie He He Hu Xi Za Zhi,
2012
Sep
, 35 (695-9).
McCrossan ZA
et al.
Regulation of the Kv2.1 potassium channel by MinK and MiRP1.
J. Membr. Biol.,
2009
Mar
, 228 (1-14).
Bocksteins E
et al.
The subfamily-specific interaction between Kv2.1 and Kv6.4 subunits is determined by interactions between the N- and C-termini.
PLoS ONE,
2014
, 9 (e98960).
Tsantoulas C
et al.
Kv2 dysfunction after peripheral axotomy enhances sensory neuron responsiveness to sustained input.
Exp. Neurol.,
2014
Jan
, 251 (115-26).
Li X
et al.
17β-Estradiol inhibits outward voltage-gated K⁺ currents in human osteoblast-like MG63 cells.
J. Membr. Biol.,
2013
Jan
, 246 (39-45).
Jamieson Q
et al.
Role of outer-pore residue Y380 in U-type inactivation of KV2.1 channels.
J. Membr. Biol.,
2013
Aug
, 246 (633-45).
Torkamani A
et al.
De novo KCNB1 mutations in epileptic encephalopathy.
Ann. Neurol.,
2014
Oct
, 76 (529-40).
Lin CH
et al.
(±)3,4-methylenedioxyamphetamine inhibits the TEA-sensitive K⁺ current in the hippocampal neuron and the Kv2.1 current expressed in H1355 cells.
Neuropharmacology,
2015
Feb
, 89 (100-12).
Kitamura N
et al.
THE INHIBITORY EFFECT OF PACLITAXEL ON (Kv2.1) K+ CURRENT IN H9c2 CELLS.
Fukushima J Med Sci,
2015
, 61 (47-53).
He K
et al.
Regulation of Pro-Apoptotic Phosphorylation of Kv2.1 K+ Channels.
PLoS ONE,
2015
, 10 (e0129498).
David JP
et al.
Auxiliary KCNE subunits modulate both homotetrameric Kv2.1 and heterotetrameric Kv2.1/Kv6.4 channels.
Sci Rep,
2015
, 5 (12813).
Cobb MM
et al.
Cell Cycle-dependent Changes in Localization and Phosphorylation of the Plasma Membrane Kv2.1 K+ Channel Impact Endoplasmic Reticulum Membrane Contact Sites in COS-1 Cells.
J. Biol. Chem.,
2015
Dec
4
, 290 (29189-201).
Aréchiga-Figueroa IA
et al.
Modulation of Kv2.1 channels inactivation by curcumin.
Pharmacol Rep,
2015
Dec
, 67 (1273-9).
Thiffault I
et al.
A novel epileptic encephalopathy mutation in KCNB1 disrupts Kv2.1 ion selectivity, expression, and localization.
J. Gen. Physiol.,
2015
Nov
, 146 (399-410).
Stas JI
et al.
Modulation of Closed-State Inactivation in Kv2.1/Kv6.4 Heterotetramers as Mechanism for 4-AP Induced Potentiation.
PLoS ONE,
2015
, 10 (e0141349).
Delgado-Ramírez M
et al.
Modulation of the voltage-gated potassium channel Kv2.1 by the anti-tumor alkylphospholipid perifosine.
Pharmacol Rep,
2016
Apr
, 68 (457-61).
Baver SB
et al.
The C-terminus of neuronal Kv2.1 channels is required for channel localization and targeting but not for NMDA-receptor-mediated regulation of channel function.
Neuroscience,
2012
Aug
16
, 217 (56-66).
Zhang YX
et al.
An exploratory study of the association between KCNB1 rs1051295 and type 2 diabetes and its related traits in Chinese Han population.
PLoS ONE,
2013
, 8 (e56365).
Shepherd AJ
et al.
Chemokine co-receptor CCR5/CXCR4-dependent modulation of Kv2.1 channel confers acute neuroprotection to HIV-1 glycoprotein gp120 exposure.
PLoS ONE,
2013
, 8 (e76698).
Guan D
et al.
Kv2 channels regulate firing rate in pyramidal neurons from rat sensorimotor cortex.
J. Physiol. (Lond.),
2013
Oct
1
, 591 (4807-25).
McCord MC
et al.
Convergent Ca2+ and Zn2+ signaling regulates apoptotic Kv2.1 K+ currents.
Proc. Natl. Acad. Sci. U.S.A.,
2013
Aug
20
, 110 (13988-93).
Windbladh B
The fate of atropine in the puppy.
Acta Pharmacol Toxicol (Copenh),
1973
, 32 (46-64).
Baver SB
et al.
Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus.
J. Neurosci.,
2014
Apr
16
, 34 (5486-96).
Romer SH
et al.
Redistribution of Kv2.1 ion channels on spinal motoneurons following peripheral nerve injury.
Brain Res.,
2013
Dec
16
, ().
King AN
et al.
A unique ion channel clustering domain on the axon initial segment of mammalian neurons.
J. Comp. Neurol.,
2014
Aug
1
, 522 (2594-608).
Kirizs T
et al.
Distinct axo-somato-dendritic distributions of three potassium channels in CA1 hippocampal pyramidal cells.
Eur. J. Neurosci.,
2014
Jun
, 39 (1771-83).
McCord MC
et al.
Syntaxin-binding domain of Kv2.1 is essential for the expression of apoptotic K+ currents.
J. Physiol. (Lond.),
2014
Aug
15
, 592 (3511-21).
Mandikian D
et al.
Cell type specific spatial and functional coupling between mammalian brain Kv2.1 K(+) channels and ryanodine receptors.
J. Comp. Neurol.,
2014
Jun
24
, ().
Siddoway B
et al.
Potassium channel Kv2.1 is regulated through protein phosphatase-1 in response to increases in synaptic activity.
Neurosci. Lett.,
2014
Nov
7
, 583 (142-7).
Pucca MB
et al.
Immunosuppressive evidence of Tityus serrulatus toxins Ts6 and Ts15: insights of a novel K(+) channel pattern in T cells.
Immunology,
2016
Feb
, 147 (240-50).
Yu Y
et al.
Association of KCNB1 polymorphisms with lipid metabolisms and insulin resistance: a case-control design of population-based cross-sectional study in Chinese Han population.
Lipids Health Dis,
2015
, 14 (112).
Fox PD
et al.
Induction of stable ER-plasma-membrane junctions by Kv2.1 potassium channels.
J. Cell. Sci.,
2015
Jun
1
, 128 (2096-105).
Angel-Chavez LI
et al.
Forskolin suppresses delayed-rectifier K+ currents and enhances spike frequency-dependent adaptation of sympathetic neurons.
PLoS ONE,
2015
, 10 (e0126365).
Wu Y
et al.
Concerted Trafficking Regulation of Kv2.1 and KATP Channels by Leptin in Pancreatic β-Cells.
J. Biol. Chem.,
2015
Dec
11
, 290 (29676-90).
Clemens K
et al.
Critical role of Casein kinase 2 in hepatitis C NS5A-mediated inhibition of Kv2.1 K(+) channel function.
Neurosci. Lett.,
2015
Nov
16
, 609 (48-52).
Saitsu H
et al.
De novo KCNB1 mutations in infantile epilepsy inhibit repetitive neuronal firing.
Sci Rep,
2015
, 5 (15199).
Contributors: Rajnish Ranjan, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/9/ , accessed on 2026 Feb 27