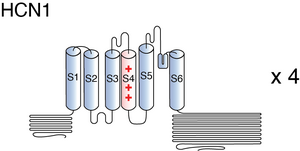
HCN1
Description: hyperpolarization-activated cyclic nucleotide-gated potassium channel 1 Gene: Hcn1 Alias: HCN1, BCNG1, HAC-2, BCNG-1, ih1
HCN1, encoded by the gene hcn1, is a hyperpolarization-activated cyclic nucleotide-gated potassium channel. HCN1 is widely expressed throughout the body, though it is highest in certain areas of the central nervous system. It is involved in the generation of the I(h) current which controls neuron excitability.
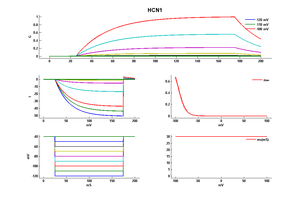
Experimental data
Rat HCN1 gene in CHO host cells |
||
|
Click for details 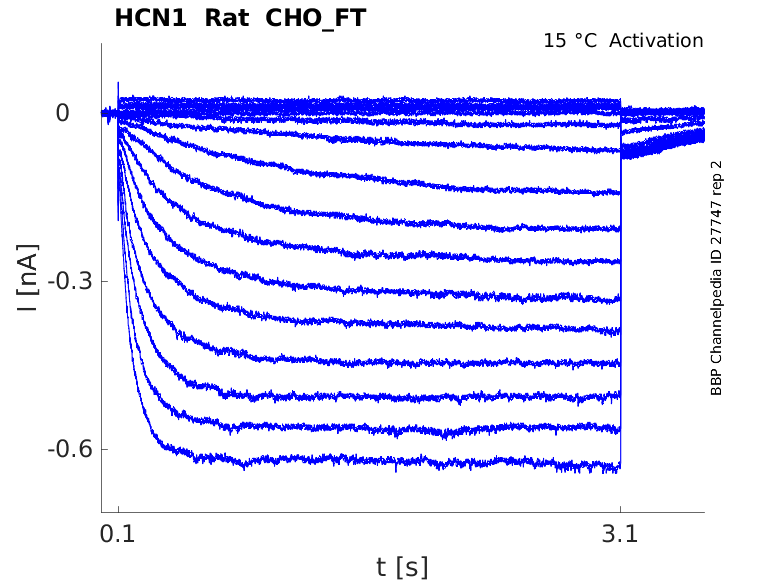
15 °Cshow 35 cells |
Click for details 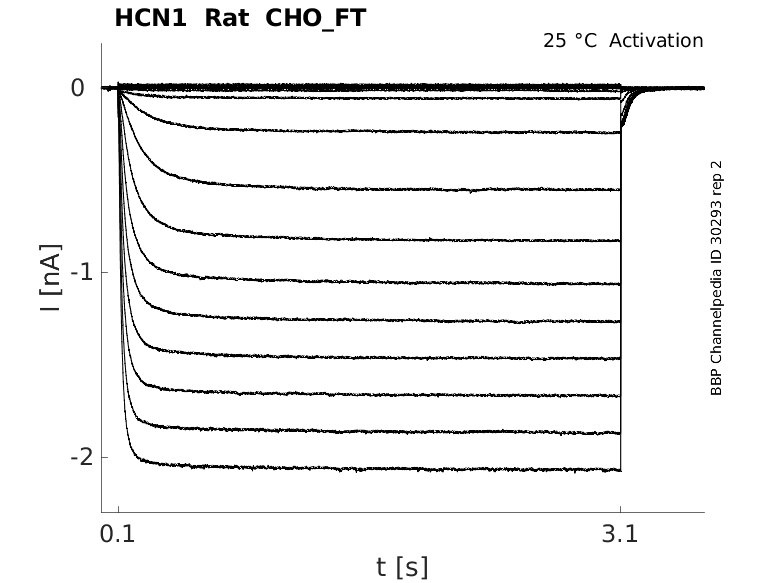
25 °Cshow 64 cells |
Click for details 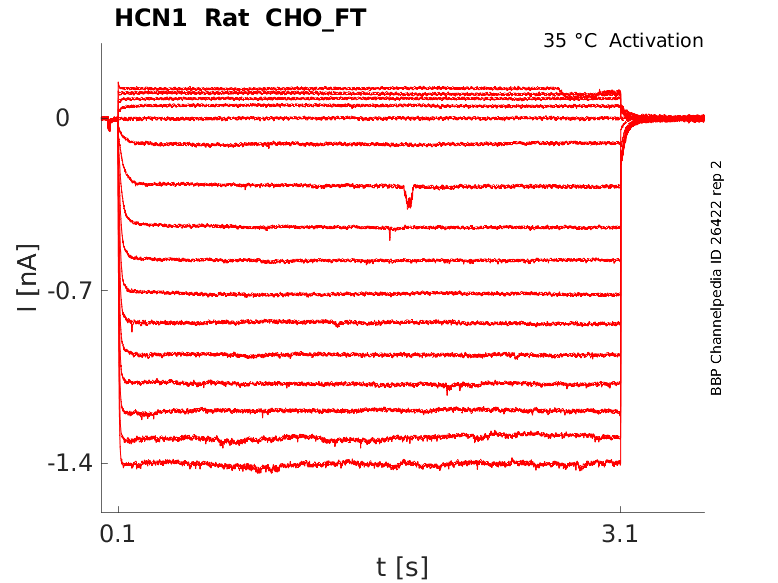
35 °Cshow 27 cells |
Mouse HCN1 gene in CHO host cells |
||
|
Click for details 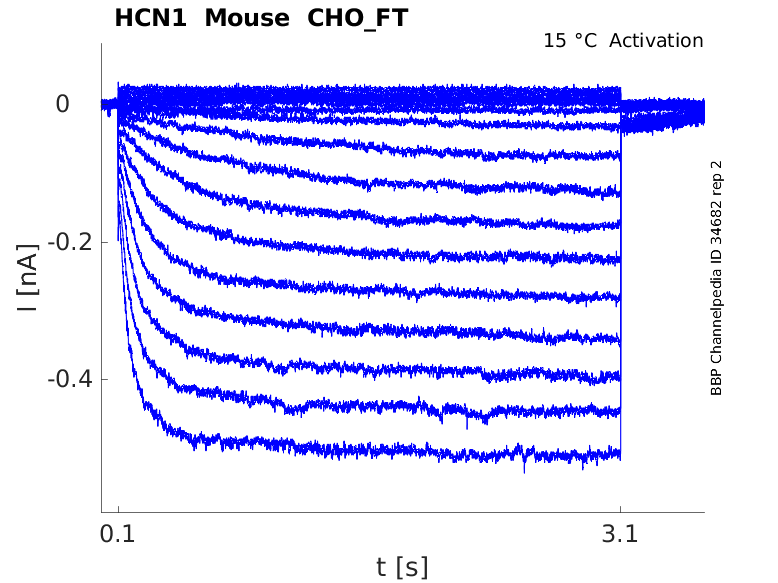
15 °Cshow 48 cells |
Click for details 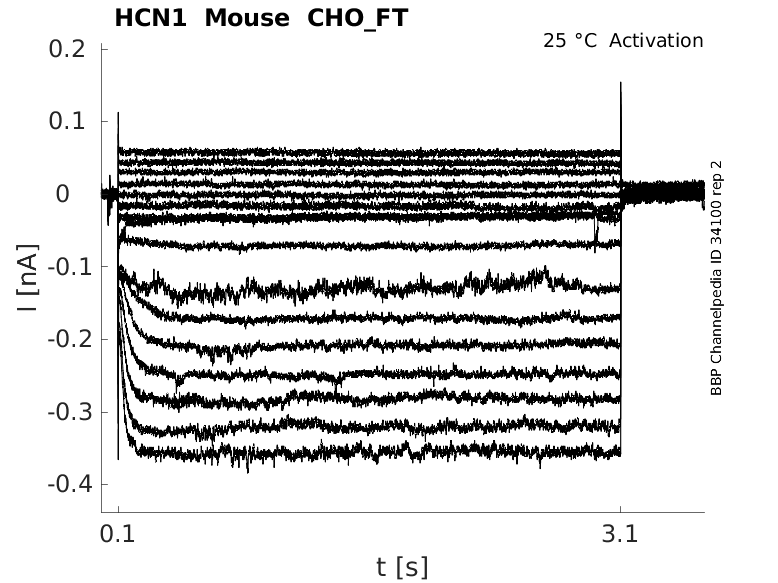
25 °Cshow 43 cells |
Click for details 
35 °Cshow 43 cells |
Human HCN1 gene in CHO host cells |
||
|
Click for details 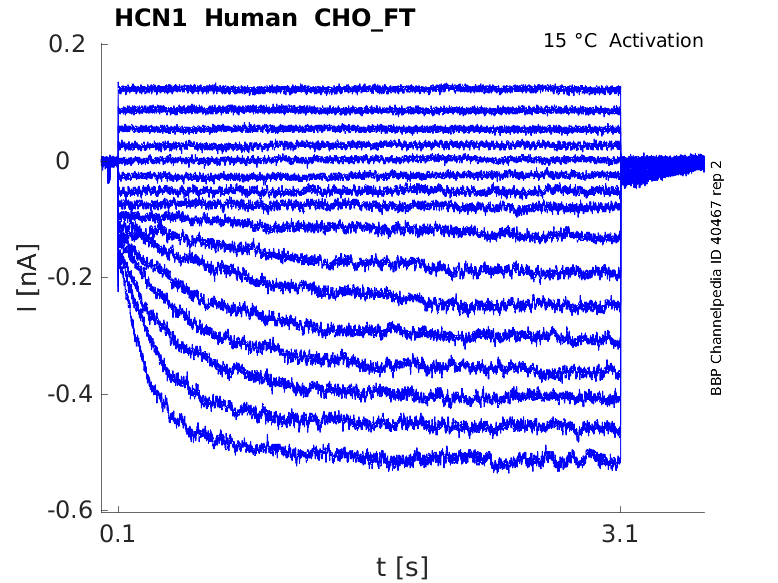
15 °Cshow 39 cells |
Click for details 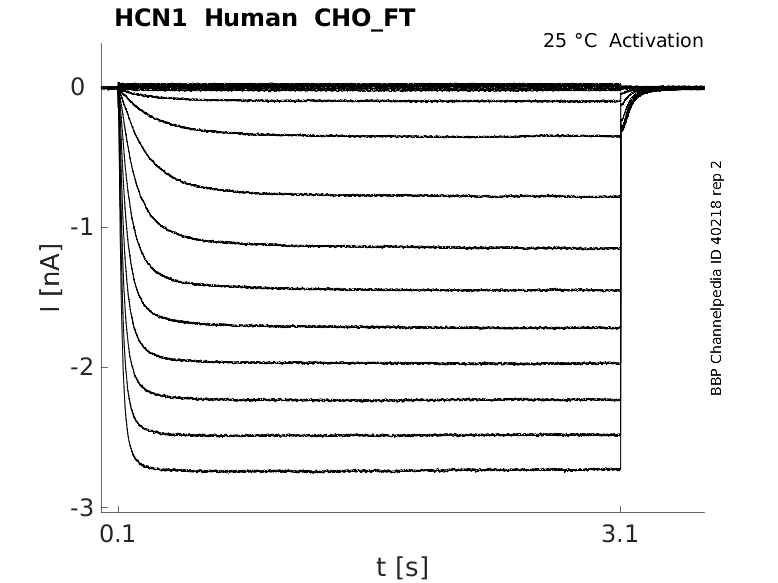
25 °Cshow 62 cells |
Click for details 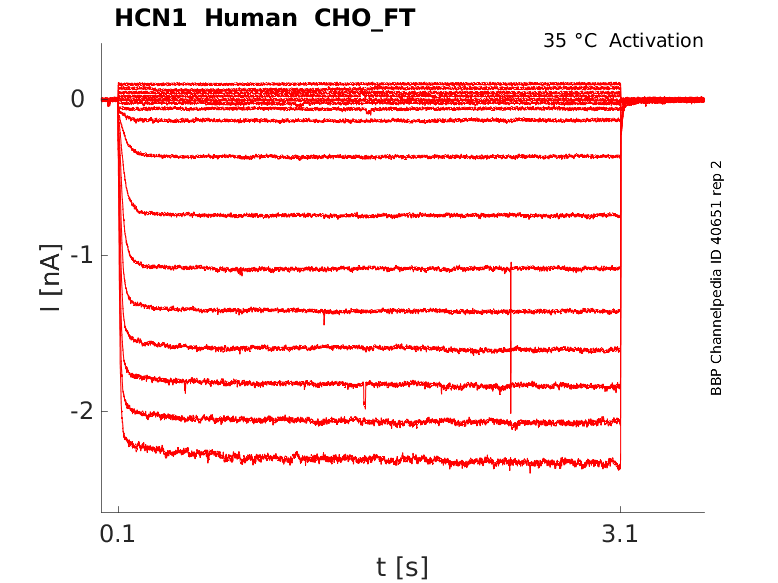
35 °Cshow 50 cells |
hcn1 is the coding gene for HCN1. In humans, hcn1 is located on chromosome 5p12 and is made up of 8 exons, all of which are coding.
To date, no transcript variants for hcn1 have been identified.
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_021072.4 | 9933 | |
| Mouse | NM_010408.3 | 7911 | |
| Rat | NM_053375.1 | 2807 |
The human HCN1 protein is composed of 890 amino acids (aa) and has a molecular weight of ~99 Kda.
To date, no isoforms resulting from alternative splicing have been identified.
Isoforms
Like most mammalian proteins, HCN1 is subject to a series of post translational modifications (PTM).
HCN1 is subject to phosphorylation by various kinases. For instance, phosphorylation by phospholipase C–protein kinase C (PKC) leads to a decrease in HCN current and a reduction in the surface expression of HCN1 [2293]. Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) activity is crucial for the proper dendritic localization of HCN1 subunits. The activation of CaMKII, triggered by upstream Ca2+ influx, results in an increase in the surface expression of HCN1 channels and a higher density of I(h) current [2295]
HCN1 undergoes ubiquitination by the Nedd4-2. This ubiquitination process results in a decrease in the cell surface expression and translocation of HCN1, ultimately leading to a loss of function in the Ih current.[2293]
HCN1, like other HCN isoforms, was also found to be S-palmitoylated in HEK293 cell experiments. However, the effects of S-palmitoylation on HCN1 have not yet been elucidated [2298].
Visual Representation of HCN1 Structure
Methodology for visual representation of structure available here
The structure of human HCN1 was resolved via cryo-electron microscopy to an overall resolution of 3.5 angstroms, giving us a detailed insight to the channel’s architecture. [2299] Structural resolution of the ion channel highlighted a number of HCN1 specific features:
- HCN is ∼100 Å in length and ∼75 Å in width
- The S4–S5 linker is much shorter and not α-helical
- The gate of HCN1 is closed by a tightly packed inner helical bundle that constricts the pore to a radius of about 1 Å
- The S4 helix is very long, containing two additional helical turns on the cytoplasmic side. The extension brings the S4–S5 linker into contact with the * C-linker of a neighboring subunit. This feature may allower the channel to stabilize a closed pore when the voltage is depolarized
The structure of the C-terminal region and changes to this area are thought to be responsible for the impact of cAMP binding to HCN channels. In the absence of cAMP, the C-terminal region is thought to exert a tonic inhibition on the pore when the C-linker and CNBD domains are in a non-tetrameric form [2303]. When the cAMP concentration increases, the C-linker/CNBD region tetramerizes and releases the inhibition from the pore gate. HCN1's C-terminal domain forms tetramers even at basal cAMP concentrations, in contrast to HCN2 and HCN4, which require saturating cAMP levels for tetramerization. This inherent tetramerization in HCN1 results in a weaker response to increased cAMP. [2304] [2304]
HCN1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
HCN1 stands out as the HCN family member with the fastest kinetics. Its activation kinetics range from 30 to 300 milliseconds at voltage levels spanning from -140 to -95 mV. Additionally, HCN1 exhibits the most positive V1/2 value, typically falling within the range of -70 to -90 mV, and initiates activation at voltages approximately 20 mV more positive than HCN2 channels.[457] [2304]
HCN1 channels behave similarly as spHCN channels with respect to voltage hysteresis behavior and mode shift. [457]
Single channel unitary conductance
Single channel unitary conductance is determined experimentally.
For HCN1, there have been recordings of single channel unitary conductance values with some degree of variance between experiments:
0.5 pS [2293]
Models
Model HCN1 (ID=9)
| Animal | Mouse | |
| CellType | Dorsal root ganglion | |
| Age | 21 Days | |
| Temperature | 0.0°C | |
| Reversal | -45.0 mV | |
| Ion | Hcn + | |
| Ligand ion | ||
| Reference | [57] S Moosmang et. al; Eur. J. Biochem. 2001 Mar | |
| mpower | 1.0 | |
| m Inf | 1.0000/(1+exp((v- -94)/8.1)) | |
| m Tau | 30.0000 | |
Cellular & Tissue
HCN1’s distribution is characterized by its very high and relatively restricted expression in both the central and peripheral nervous system [2306].
In the CNS, these areas are:
- Neocortex [457]
- Hippocampus [2307]
- Cerebellum [2307]
- Selected nuclei of the brain stem [2308]
- Olfactory bulb [338]
- Superior colliculus [323]
- Facial motor nucleus [337]
Within these various regions, HCN1 levels can vary greatly. HCN1 mRNA expression was demonstrated to be at least eightfold higher in cortical neurons than subcortical neurons [2309]. Two neuronal classes stand out for their exceptionally high HCN1 levels: cortical pyramidal neurons and parvalbumin-positive (PV+) interneurons (basket cells) in the cortex and cerebellum.[2306]
HCN1 was also identified in the PNS. Identified areas include:
- Nucleus laminaris (NL) region [2310]
- A-type neurons [2311]
- Mechanosensitive terminals of myelinated sensory fibers [2311]
- Large neurons I(h) [2312]
- Atrioventricular node of the heart [457]
For a more detailed map immunohistochemical localisation of HCN4, please consult the following paper [323]
Developmental
HCN1 was identified at low levels in early development (P1 and P5) but steadily increased by P20. [2313] [2314] Indeed, HCN1 experiences a number of changes in not only its expression but also its distribution over the course of development. In rodents, HCN1 channels tend to shift to dendrites soon after birth. In certain regions, like the hippocampus, HCN1 first appears in the axon terminals but disappears as maturity sets in. These changes in distribution are mediated by accessory proteins involved in channel trafficking [2295].
The subcellular localization of HCN channels is neuron-type-specific. It is therefore difficult to generalize and pinpoint the exact cellular locations of these channels.
In hippocampal and cortical pyramidal cells, HCN1 can be found in the distal dendrites. In other pyramidal neurons, HCN1 is also located in the somata, albeit at a lower density than that in distal dendrites. [2307] Quantitative analysis revealed a 60-fold increase in the presence of HCN1 channels from somatic to distal apical dendritic membranes. Moreover, distal dendritic shafts exhibited 16 times more HCN1 labeling compared to proximal dendrites with similar diameters. Additionally, at the same distance from the cell body, the density of HCN1 channels was significantly higher in dendritic shafts than in dendritic spines.[324]
In the interneurons of medial septum, hippocampus and cerebellum, HCN1, along with other HCN channels, are expressed at somatic and axonal regions [2295] [321]. HCN1 has also been found present at axonal terminals and preterminal axons of hippocampal basket cells. [2306] In cerebellar basket cells, HCN1 is present at a low density in the somato-dendritic regions but is present at slightly higher density in axon terminals. [321] The presence of HCN1 channels in synaptic terminals, both excitatory and inhibitory if thought to regulate basal glutamatergic synaptic release, impacting overall synaptic transmission. [2307]
Learning and memory
Deletion of HCN1 has been demonstrated to result in notable impairments in motor skills and memory tasks. Targeted knock-out experiments conducted in Purkinje cerebellar cells have highlighted the stabilizing role of HCN1 within these cells. HCN1 plays a critical role in maintaining the reliable encoding of information and the accurate decoding of input patterns, contributing to proper motor function and memory processes. [457] [2315]
HCN1's contribution to learning and memory is further demonstrated through region-specific knockout experiments. HCN1 deletion from forebrain neurons was shown to have a profound effect on hippocampal-dependent learning and memory processes. This manipulation amplifies theta oscillations and boosts long-term potentiation (LTP) specifically at the input to the distal dendrites of CA1 pyramidal neurons. The underlying mechanism for this influence on learning is attributed to HCN1's regulatory role in the dendritic integration of distal synaptic inputs within pyramidal cells.[2316] [457]
Nociception
HCN1 is thought to contribute to nociception as blockade of the channel via inhibitors was shown to decrease light hypersensitivity in rat model experiments. [2317] Indeed, genetic deletion or pharmacological inhibition of HCN1 generally provides partial analgesia to certain types of neuropathic pain, such as cold hypersensitivity in most animal pain models [2318] [2312]
Channelopathies
HCN1 have been associated with a number of pathologies with epilepsy being the most common channelopathy associated with deregulation of the channel [2319]. Conditions include different gain of function and loss of function epilepsy types, like common forms but also others such as intractable epileptic encephalopathy, temporal lobe epilepsy and absence seizures [2320] [2321] [2322]
HCN1 is also thought to impact affective disorders. HCN1 knockout mice displayed antidepressant-like behavior, highlighting the potential role of HCN1 as a target for anxiety and depression disorders. [2323]
Heteromeric channels
However, there is evidence that certain HCN channels, coexpressed in the same regions, coassemble to generate heteromultimeric channels. For example, HCN1 can be found in conjunction with HCN2, HCN3, and HCN4 in certain areas, leading to the formation of hybrids consisting of both HCN1 and HCN2/HCN3/HCn4 subunits. These heteromultimeric isoforms of HCN channels exhibit I(h) currents with activation kinetics and voltage dependence that tend to fall in between those of the "parent" homomers. These heteromers also display a relatively large shift by cAMP (+14 mV) [1697] [2296].
HCN1, like other channels, is regulated by various auxiliary proteins and secondary messengers. These regulatory proteins play important roles in the development, localization, and expression of the channel protein.
Unlike HCN2 and HCN4, HCN1 is insensitive to cyclic nucleotides, such as cAMP or cGMP. The reason for this lack of modulation is due to structural differences between the individual channels, further explained in the Structure section.
Filamin A is a cytoplasmic scaffold protein with actin-binding domains whose main function is to link transmembrane proteins to the actin cytoskeleton. Filamin A is known to interact with HCN1 and contributes to localizing the channel to specific neuronal areas and slows down activation and deactivation kinetics. [2324] [457]
Sinus node inhibitors such as cilobradine, ivabradine, and zatebradine are known blockers for HCN channels often used as treatment within the context of heart disease. All three substances block the slow inward current through human HCNs. For HCN1, the blockade happened in a 'closed-channel' state. (16484306) [2305]
Cannabinoids have also shown interaction with HCN1 and are thought to impact long-term potentiation (LTP), and spatial memory formation in the hippocampus [2325].
HCN1-selective compounds have been described [2326]
TRIP8 is brain-specific cytoplasmic protein, of which nine isoform were identified. TRIP8b is known to interact with all HCN, the results of the interactions depending on the type of HCN and TRIP8b isoform involved. TRIP8b (1a–4) and TRIP8b (1a), are major splice variants presen in the hippocampus. They enable a correct localization of HCN1, upregulate HCN1 expression in heterologous systems and promote its dendritic expression. Conversely, TRIP8b (1a) downregulates HCN1 surface expression in X. laevis oocytes and inhibits the abnormal expression of HCN1 in the axons of pyramidal neurons [2293]
For further compounds interactions, please consult the following resource
References
Brandt MC
et al.
Effects of KCNE2 on HCN isoforms: distinct modulation of membrane expression and single channel properties.
Am. J. Physiol. Heart Circ. Physiol.,
2009
Jul
, 297 (H355-63).
Moosmang S
et al.
Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues.
Eur. J. Biochem.,
2001
Mar
, 268 (1646-52).
Nusser Z
Variability in the subcellular distribution of ion channels increases neuronal diversity.
Trends Neurosci.,
2009
May
, 32 (267-74).
Notomi T
et al.
Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain.
J. Comp. Neurol.,
2004
Apr
5
, 471 (241-76).
Lorincz A
et al.
Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites.
Nat. Neurosci.,
2002
Nov
, 5 (1185-93).
Monteggia LM
et al.
Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain.
Brain Res. Mol. Brain Res.,
2000
Sep
30
, 81 (129-39).
Moosmang S
et al.
Differential distribution of four hyperpolarization-activated cation channels in mouse brain.
Biol. Chem.,
1999 Jul-Aug
, 380 (975-80).
Biel M
et al.
Hyperpolarization-activated cation channels: a multi-gene family.
Rev. Physiol. Biochem. Pharmacol.,
1999
, 136 (165-81).
Giorgetti A
et al.
A homology model of the pore region of HCN channels.
Biophys. J.,
2005
Aug
, 89 (932-44).
Park K
et al.
HCN channel activity-dependent modulation of inhibitory synaptic transmission in the rat basolateral amygdala.
Biochem. Biophys. Res. Commun.,
2011
Jan
28
, 404 (952-7).
Biel M
et al.
Hyperpolarization-activated cation channels: from genes to function.
Physiol. Rev.,
2009
Jul
, 89 (847-85).
Meng QT
et al.
LOCAL ANESTHETIC INHIBITS HYPERPOLARIZATION ACTIVATED CATIONIC CURRENTS.
,
2011
Feb
8
, ().
Benarroch EE
HCN channels: function and clinical implications.
Neurology,
2013
Jan
15
, 80 (304-10).
Zhang Z
et al.
The APC/C subunit Cdc16/Cut9 is a contiguous tetratricopeptide repeat superhelix with a homo-dimer interface similar to Cdc27.
EMBO J.,
2010
Nov
3
, 29 (3733-44).
Tanimoto N
et al.
HCN1 channels significantly shape retinal photoresponses.
Adv. Exp. Med. Biol.,
2012
, 723 (807-12).
Chen S
et al.
Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide.
J. Gen. Physiol.,
2001
May
, 117 (491-504).
Sartiani L
et al.
The Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels: from Biophysics to Pharmacology of a Unique Family of Ion Channels.
Pharmacol Rev, 2017Oct, 69 (354-395).
He C
et al.
Neurophysiology of HCN channels: From cellular functions to multiple regulations.
Prog. Neurobiol.,
2014
Jan
, 112 (1-23).
Much B
et al.
Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels.
J. Biol. Chem.,
2003
Oct
31
, 278 (43781-6).
Itoh M
et al.
The hyperpolarization-activated cyclic nucleotide-gated (HCN) channels contain multiple S-palmitoylation sites.
J Physiol Sci, 2016May, 66 (241-8).
Lee CH
et al.
Structures of the Human HCN1 Hyperpolarization-Activated Channel.
Cell, 2017Jan12, 168 (111-120.e11).
Wainger BJ
et al.
Molecular mechanism of cAMP modulation of HCN pacemaker channels.
Nature,
2001
Jun
14
, 411 (805-10).
Ulens C
et al.
Regulation of hyperpolarization-activated HCN channels by cAMP through a gating switch in binding domain symmetry.
Neuron,
2003
Dec
4
, 40 (959-70).
Lolicato M
et al.
Tetramerization dynamics of C-terminal domain underlies isoform-specific cAMP gating in hyperpolarization-activated cyclic nucleotide-gated channels.
J. Biol. Chem.,
2011
Dec
30
, 286 (44811-20).
Stieber J
et al.
Bradycardic and proarrhythmic properties of sinus node inhibitors.
Mol Pharmacol, 2006Apr, 69 (1328-37).
Santoro B
et al.
Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels as Drug Targets for Neurological Disorders.
Annu Rev Pharmacol Toxicol, 2020Jan06, 60 (109-131).
Shah MM
Cortical HCN channels: function, trafficking and plasticity.
J. Physiol. (Lond.),
2014
Jul
1
, 592 (2711-9).
Santoro B
et al.
Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS.
J. Neurosci.,
2000
Jul
15
, 20 (5264-75).
Franz O
et al.
Single-cell mRNA expression of HCN1 correlates with a fast gating phenotype of hyperpolarization-activated cyclic nucleotide-gated ion channels (Ih) in central neurons.
Eur. J. Neurosci.,
2000
Aug
, 12 (2685-93).
Yamada R
et al.
Hyperpolarization-activated cyclic nucleotide-gated cation channels regulate auditory coincidence detection in nucleus laminaris of the chick.
J. Neurosci.,
2005
Sep
28
, 25 (8867-77).
Doan TN
et al.
Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers.
J. Neurosci.,
2004
Mar
31
, 24 (3335-43).
Momin A
et al.
Role of the hyperpolarization-activated current Ih in somatosensory neurons.
J. Physiol. (Lond.),
2008
Dec
15
, 586 (5911-29).
Vasilyev DV
et al.
Postnatal development of the hyperpolarization-activated excitatory current Ih in mouse hippocampal pyramidal neurons.
J. Neurosci.,
2002
Oct
15
, 22 (8992-9004).
Kanyshkova T
et al.
Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations.
J. Neurosci.,
2009
Jul
8
, 29 (8847-57).
Nolan MF
et al.
The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells.
Cell,
2003
Nov
26
, 115 (551-64).
Nolan MF
et al.
A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons.
Cell,
2004
Nov
24
, 119 (719-32).
Chaplan SR
et al.
Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain.
J. Neurosci.,
2003
Feb
15
, 23 (1169-78).
Lainez S
et al.
HCN3 ion channels: roles in sensory neuronal excitability and pain.
J Physiol, 2019Sep, 597 (4661-4675).
Kessi M
et al.
The Contribution of HCN Channelopathies in Different Epileptic Syndromes, Mechanisms, Modulators, and Potential Treatment Targets: A Systematic Review.
Front Mol Neurosci, 2022, 15 (807202).
Budde T
et al.
Impaired regulation of thalamic pacemaker channels through an imbalance of subunit expression in absence epilepsy.
J. Neurosci.,
2005
Oct
26
, 25 (9871-82).
Nava C
et al.
De novo mutations in HCN1 cause early infantile epileptic encephalopathy.
Nat. Genet.,
2014
Jun
, 46 (640-5).
McClelland S
et al.
Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy.
Ann. Neurol.,
2011
Sep
, 70 (454-64).
Kim CS
et al.
Enhancement of dorsal hippocampal activity by knockdown of HCN1 channels leads to anxiolytic- and antidepressant-like behaviors.
Neuron,
2012
Aug
9
, 75 (503-16).
Gravante B
et al.
Interaction of the pacemaker channel HCN1 with filamin A.
J. Biol. Chem.,
2004
Oct
15
, 279 (43847-53).
Maroso M
et al.
Cannabinoid Control of Learning and Memory through HCN Channels.
Neuron, 2016Mar02, 89 (1059-73).
McClure KJ
et al.
Discovery of a novel series of selective HCN1 blockers.
Bioorg. Med. Chem. Lett.,
2011
Sep
15
, 21 (5197-201).
Contributors: Katherine Johnston, Rajnish Ranjan, Michael Schartner, Nitin Khanna
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/61/ , accessed on 2025 Dec 30