Kv1.6
Description: potassium voltage gated channel, shaker related subfamily, member 6 Gene: Kcna6 Alias: Kv1.6, kcna6, MK1.6
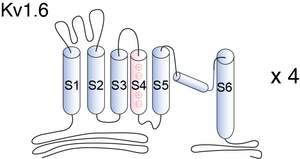
Kv1.6, encoded by the gene KCNA6, is a member of the potassium voltage-gated channel subfamily A. Kv1.6 contains six membrane-spanning domains with a shaker-type repeat in the fourth segment. These subunits also behave as ‘delayed rectifiers’ in homomeric assemblies. NCBI source
Experimental data
Rat Kv1.6 gene in CHO host cells datasheet |
||
|
Click for details 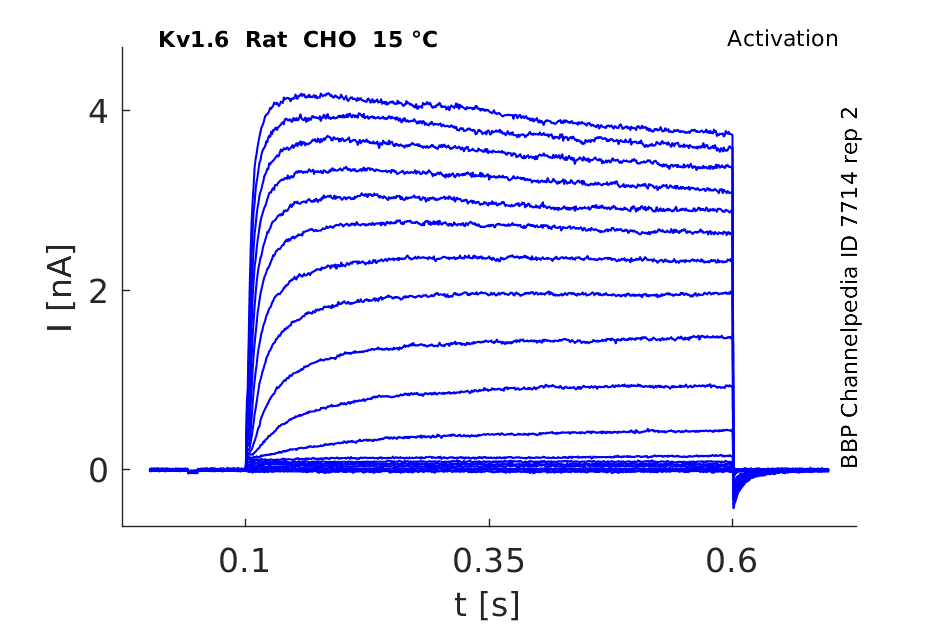
15 °Cshow 50 cells |
Click for details 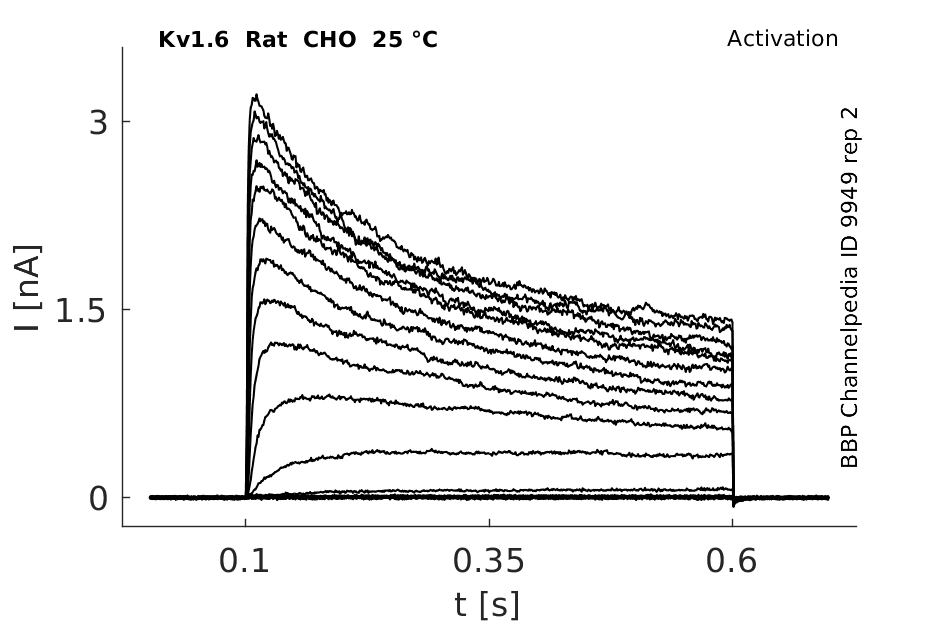
25 °Cshow 123 cells |
Click for details 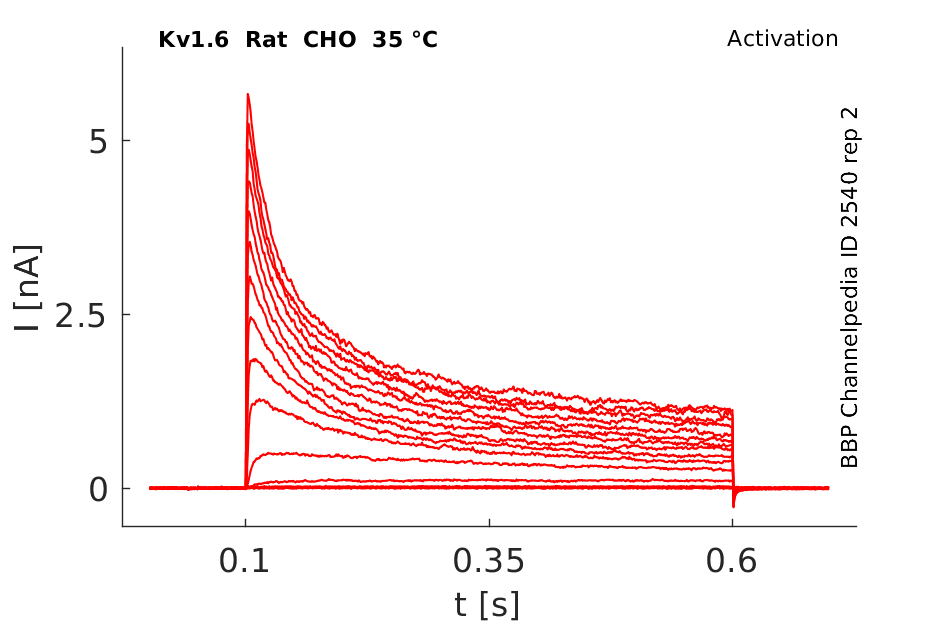
35 °Cshow 59 cells |
Mouse Kv1.6 gene in CHO host cells datasheet |
||
|
Click for details 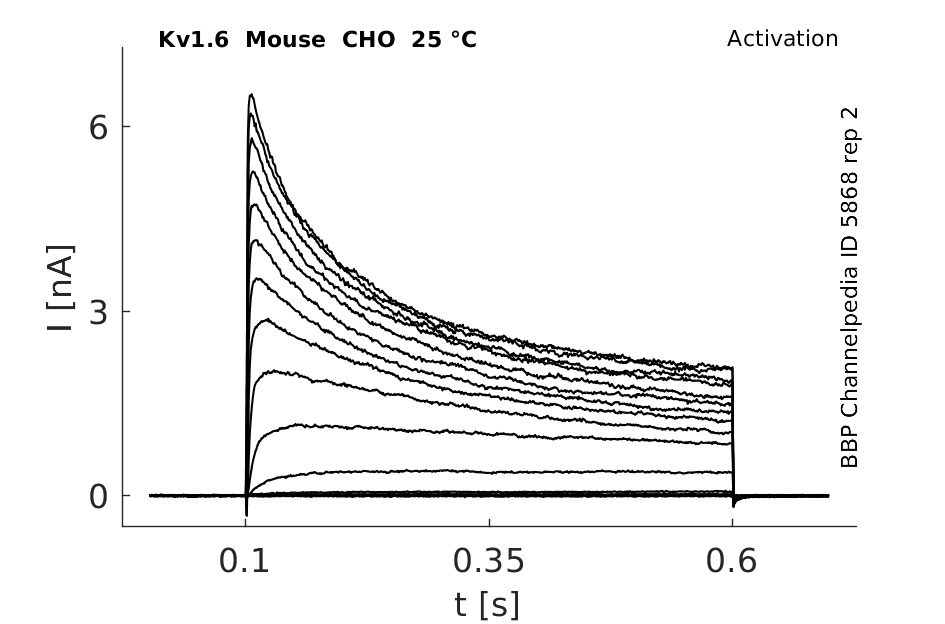
25 °Cshow 44 cells |
Click for details 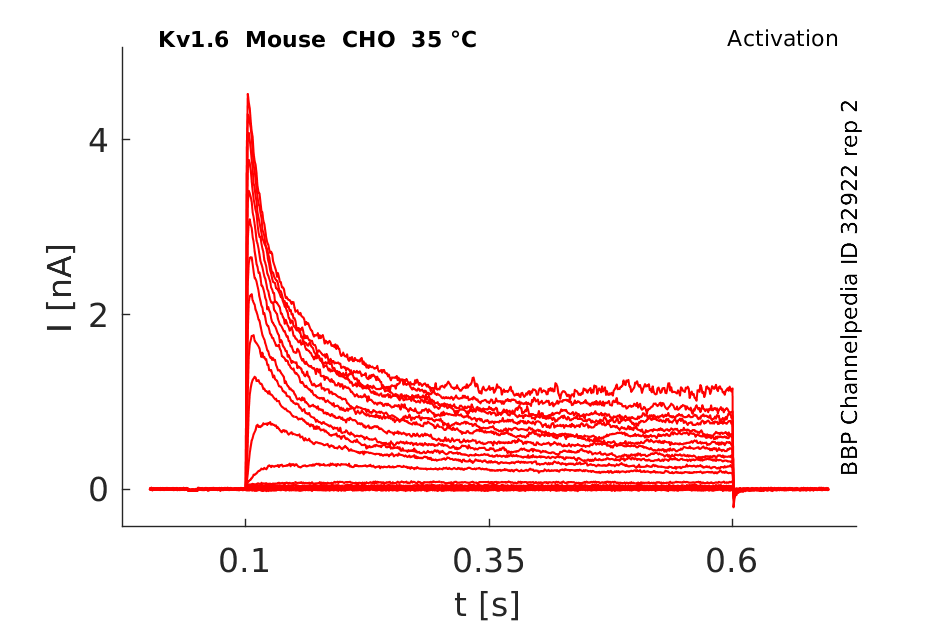
35 °Cshow 37 cells |
|
Human Kv1.6 gene in CHO host cells datasheet |
||
|
Click for details 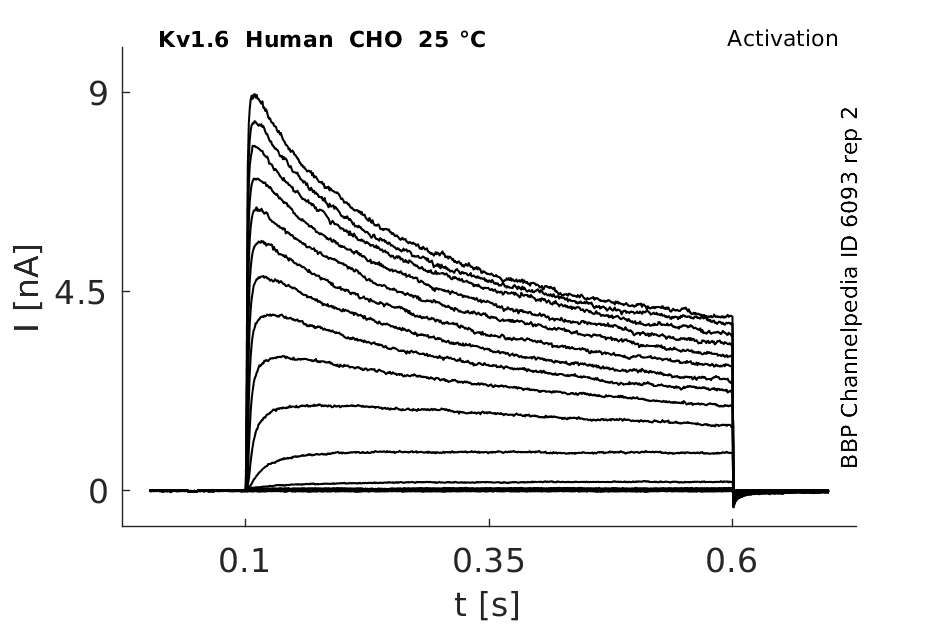
25 °Cshow 81 cells |
Click for details 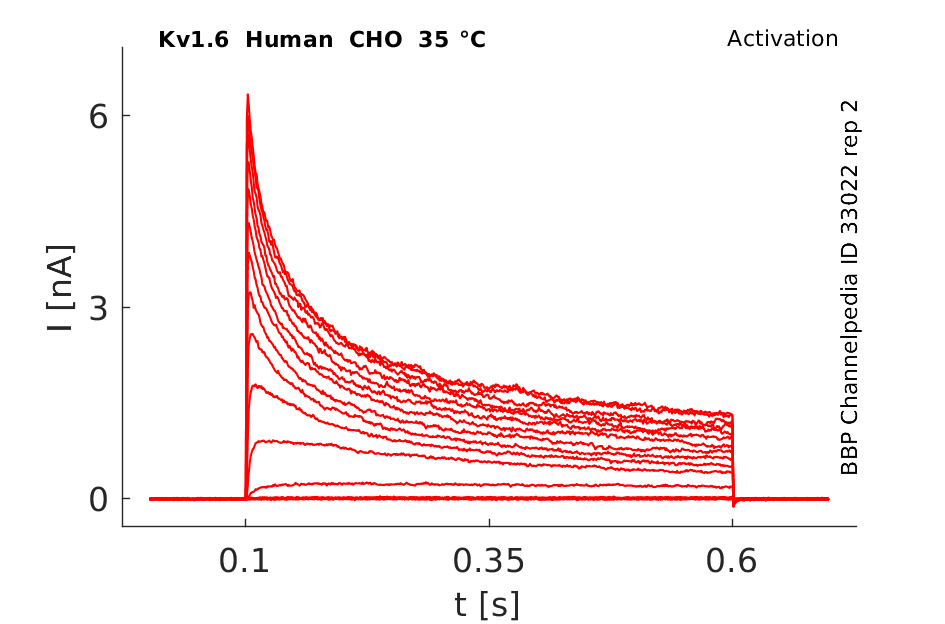
35 °Cshow 141 cells |
|
Rat Kv1.6 gene in HEK host cells datasheet |
||
|
Click for details 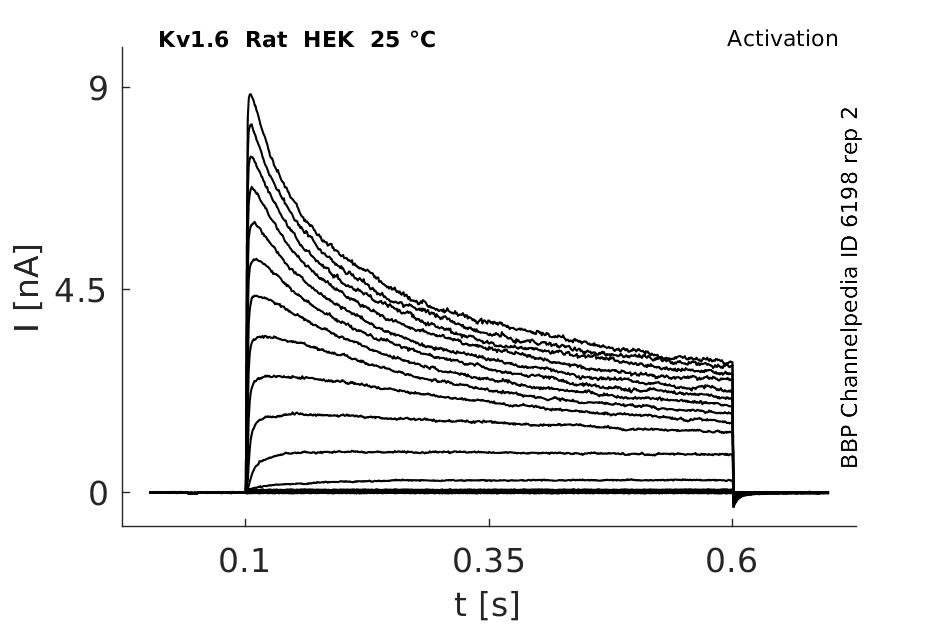
25 °Cshow 81 cells |
Click for details 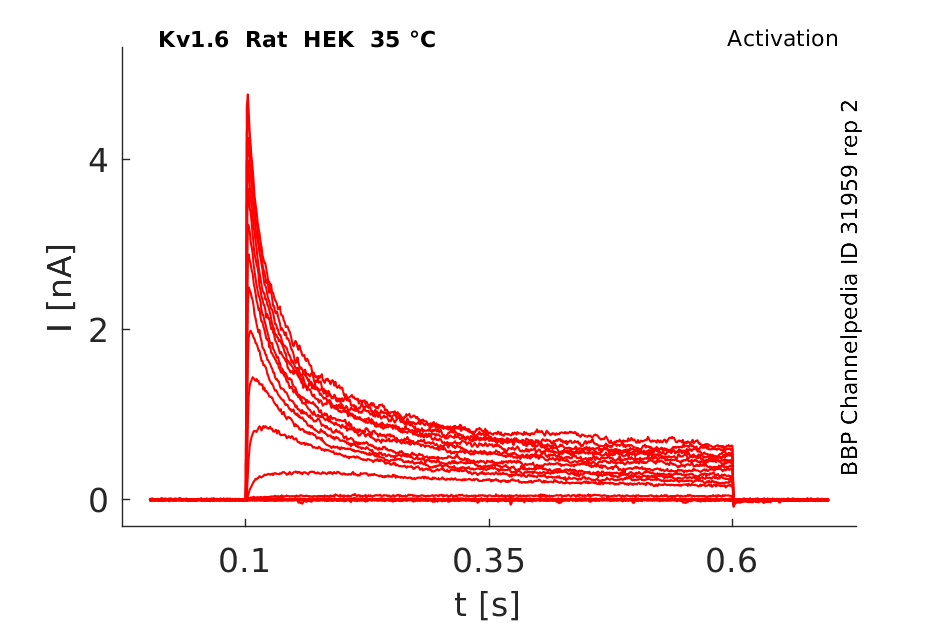
35 °Cshow 61 cells |
|
Rat Kv1.6 gene in CV1 host cells datasheet |
||
|
Click for details 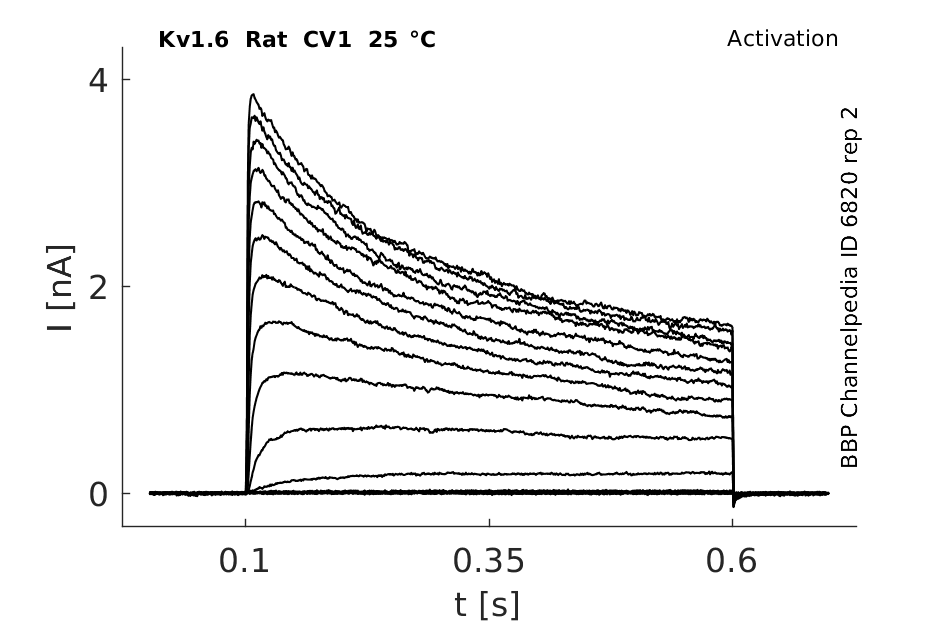
25 °Cshow 220 cells |
Click for details 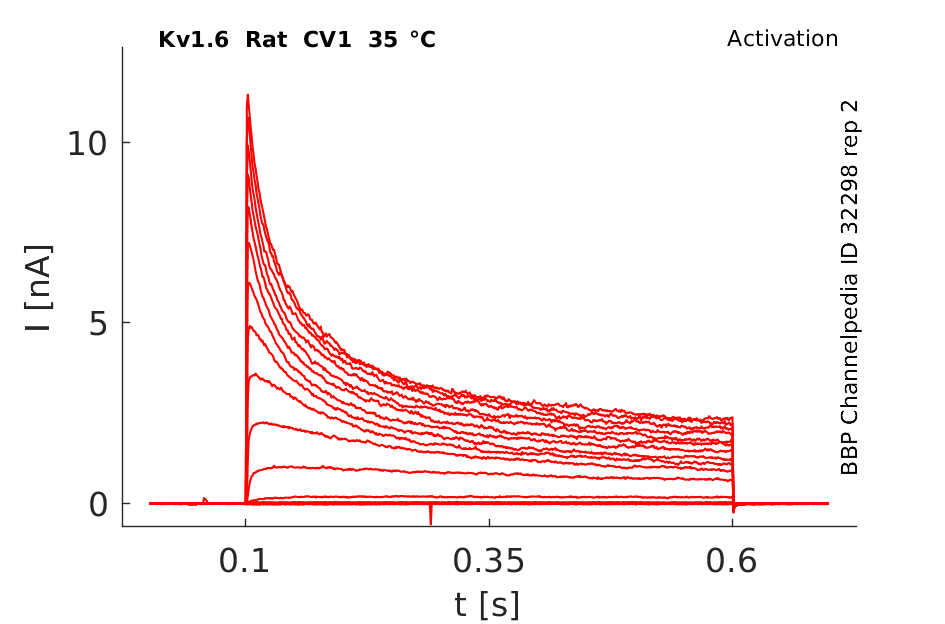
35 °Cshow 72 cells |
|
The coding region of this gene is intronless, and the gene is clustered with genes KCNA1 and KCNA5 on chromosome 12.
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_002235.5 | 3985 | |
| Mouse | NM_013568.6 | 5853 | |
| Rat | NM_023954.2 | 6696 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv1.6 Structure
Methodology for visual representation of structure available here
Crystal Structure

Kv1.6 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
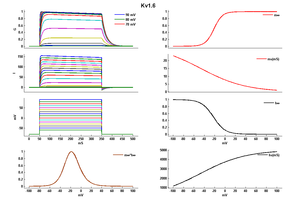
Kv1.6 kinetics
In vitro expression of this channel produces an outward current with an activation threshold around 240 mV and little inactivation during a 200 ms pulse [1620]
Voltage-gated K channels, Kvl.1, Kvl.4, Kvl.5, and Kvl.6, cloned from rat brain and expressed in Xenopus oocytes have similar activation and deactivation kinetics. [431]
The single-channel conduc- tance of Kv1.4 is much smaller than that of either Kv1.1 or Kv1.6 [1621]
Kv1.6 currents measured in Xenopus oocytes and effect of Bcstx2
Xenopus oocytes expressing Kv1.6 channels were used to record whole-cell currents. After 500 ms depolarizations to 0 mV, a 500 ms pulse to −50 mV was used to induce IR currents, from a holding potential of −90 mV. The scorpion toxin Bcstx2 potently inhibited rKv1.6 currents.[2043]
Biophysics
Model Kv1.6 (ID=22)
| Animal | Xenopus | |
| CellType | oocyte | |
| Age | 0 Days | |
| Temperature | 23.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [276] O Pongs et. al; EMBO J. 1990 Jun | |
| mpower | 1.0 | |
| m Inf | 1/(1+exp(((v -(-20.800))/(-8.100)))) | |
| m Tau | 30.000/(1+exp(((v -(-46.560))/(44.140)))) | |
| hpower | 1.0 | |
| h Inf | 1/(1+exp(((v -(-22.000))/(11.390)))) | |
| h Tau | 5000.000/(1+exp(((v -(-46.560))/(-44.140)))) | |
Kv1.6 appeared uniform throughout the bovine brain. Kv-specific antibody precipitated a different proportion (anti-Kvl.2 > 1.1 >> 1.6 > 1.4) of the channels detectable with radioiodinated alpha-DTX in every brain region.[432]
Kv1.6 was found in human cardiac fibroblasts, besides more numerous other ion-channels [428] and also in in murine colon myocytes [430].
All the a-subunits of Kv1.1–Kv1.6 channels were found to be expressed in the ganglion cell layer (GCL) of the rat retina at postnatal day 5 (P5). The GCL expressed Kv1.6 channels already at P1 at high levels. Their expression upregulates postnatally and the pattern and distribution change in an isoform-specific manner.[429]
Kv1.6 transcript was found in astrocyte cultures at a level approximately 65% in primary cultures and 16% in secondary cultures of the level found in adult mouse brain [1620]
MNTB
Immunohistochemistry confirms that Kv1.1 and Kv1.2 (ILTS channels), but not Kv1.6, are concentrated in the first 20 um of the medial nucleus of the trapezoid body axons. Kv1.6 immunofluorescence showed somatic but not axonal staining was observed (n= 4 animals) [1704]
Subunit-specific trafficking
Kv1 channels are trafficked in a subunit-specific manner. Kv1.6 is predominantly localized to the endoplasmic reticulum in astrocytes while Kv1.3 is localized to the cis-Golgi [1978]
Excitability of Sensory Neurons
Potassium channels Kv1.1, Kv1.2 and Kv1.6 influence excitability of rat visceral sensory neurons [1792]
A modification of the biophysical properties of Kv1.6-induced K+ current is triggered by the expression level of Kv1.6 channel in the cell membrane. The inactivation properties are close to canonical C-type inactivation.[11]
Synaptic Transmission
Besides being responsible for cell membrane repolarization after initiation of an action potential, Kv1.6 - like other voltage-gated K+ channels - is known to modulate synaptic transmission and secretion from endocrine cells, such as insulin from pancreatic islet cells [590]. The basic electrophysiological characteristics (i.e., kinetics) of Kv channels contribute importantly to the modulation of the shape, frequency, and duration of the cardiac action potential [591], [592].
The fast N-type inactivation endowed by Kv1.4 can be prevented by the presence of Kv1.6 through its NIP (N-type inactivation prevention) domain, giving rise to a much slower inactivating/sustained K+ current [380]
DISEASE
Amyotrophic Lateral Sclerosis
CSF from Amyotrophic Lateral Sclerosis (ALS) patients (ALS-CSF) has the potential to perturb ion channel expression, specifically the Na(v)1.6, and K(v)1.6 channels in newborn rat spinal motor neurons both in vivo and in vitro. A decrease in the expression of Na(v)1.6 and K(v)1.6 channels in motor neurons in ALS-CSF treated group, and the presence of trophic factors like Brain Derived Neurotrophic Factor (BDNF) and Ciliary Neurotrophic Factor CNTF partially reversed the effects produced by ALS-CSF [381]
Nociception
Kv channels might be involved in the failure of polymodal nociceptive C-fibers associated with diabetic hyperalgesia. α-Dendrotoxin (α-DTX) down-regulates Kv1.2 and Kv1.6 channel currrents in small dorsal root ganglion neurons and C-fibers. Malfunction of α-DTX-sensitive Kv1 channels may therefore be involved in the hyperexcitability of these neurons [1934]
Vasodilatation
The cholinergic regulation of arterial luminal diameter involves a functionally relevant role of Kv1.6 channels. Kv1.6 channels were localized exclusively in the smooth muscle cells of the mouse ophthalmic artery [2045]
Mg2+
Kv1.4 channels are more sensitive to block by internal Mg2+ at low depolarizations than either Kv1.1 or Kv1.6.
TEA
The mutation of Kv1.4K533 at the putative outer mouth of the pore to Y, the equivalent residue in Kv1.1, has already been shown to greatly increase the sensitivity of Kv1.4 channels to external TEA+, such that it is the same as that of Kv1.1. We have now found that substitu- tion of the corresponding tyrosine (Y430) in Kv1.6 by lysine produces, as expected from the effect of the con- verse mutation in Kv1.4, a massive (at least 35-fold) de- crease in the sensitivity of Kv1.6 channels to external TEA+
Strontium
Strontium (Sr2+; 7-50 mM) effects Kv1.6 (from rat, expressed in Xenopus oocytes) in so far as the K+ conductance curve is shifted along the potential axis, where the shift of Kv1.6 lies between that of Kv1.1, was shifted most and Kv3.4, least, 21 and 8 mV, respectively, at 50 raM. [12]
Kv1.4
Subunit ordering of Kv1 channels influences their properties: N-type rapid deactivation of Kv1.4 containing channels is over-ridden when Kv1.6 subunits are adjacent to the Kv1.4 subunit. Placing Kv1.4 and Kv1.6 genes together, followed by two copies of Kv1.2, prevents the K+ current from fast inactivation. In contrast, separating Kv1.4 and Kv1.6 by a copy of Kv1.2 genes between them, fast inactivation is prevented again.[380]
Kv-beta-3
Kvbeta3 confered a rapid inactivation to Kv1.6 channels. Kv1.6 channels that possess an N-type inactivation prevention (NIP) domain for Kvbeta1.1, inactivated rapidly when co-expressed with Kvbeta3. [390]
Dendrotoxins
The dendrotoxins have little or no anti-protease activity, but they were demonstrated to block particular subtypes of voltage-dependent potassium channels in neurons. Studies with cloned K(+) channels indicate that alpha-dendrotoxin from green mamba Dendroaspis angusticeps blocks Kv1.1, Kv1.2 and Kv1.6 channels in the nano molar range [1618]
Toxin Blockers
Charybdotoxin, margatoxin and hongotoxin inhibit Kv1.6 in the low nM range. Another scorpion toxin, tamulustoxin (from the indian red scorpion, Methobuthus tamales) has been recently identified as a blocker of Kv1.6 channels expressed in CHO cells.
(http://www.tocris.com/pdfs/pdfdownloads/potassiumreview.pdf)
pl14a in X oocytes

Sea anemone toxin BcsTx2
Sea anemone neurotoxins can be useful to elucidate the function of voltage-gated K+ channels (KV). A comparitive study of novel Kv1 channel toxins demostrated that BcsTx1 shows high affinity for rKv1.2 while BcsTx2 potently blocked rKv1.6 [2043]
References
Guihard G
et al.
Human Kv1.6 current displays a C-type-like inactivation when re-expressed in cos-7 cells.
Biochem. Biophys. Res. Commun.,
2003
Nov
7
, 311 (83-9).
Elinder F
et al.
Surface Charges of K channels. Effects of strontium on five cloned channels expressed in Xenopus oocytes.
J. Gen. Physiol.,
1996
Oct
, 108 (325-32).
Grupe A
et al.
Cloning and expression of a human voltage-gated potassium channel. A novel member of the RCK potassium channel family.
EMBO J.,
1990
Jun
, 9 (1749-56).
Bodeker M
et al.
Position-dependent attenuation by Kv1.6 of N-type inactivation of Kv1.4-containing channels.
,
2011
Feb
25
, ().
Gunasekaran R
et al.
Exposure to cerebrospinal fluid of sporadic amyotrophic lateral sclerosis patients alters Nav1.6 and Kv1.6 channel expression in rat spinal motor neurons.
Brain Res.,
2009
Feb
19
, 1255 (170-9).
Bähring R
et al.
Differential modulation of Kv1 channel-mediated currents by co-expression of Kvbeta3 subunit in a mammalian cell-line.
Mol. Membr. Biol.,
2004 Jan-Feb
, 21 (19-25).
Li GR
et al.
Characterization of multiple ion channels in cultured human cardiac fibroblasts.
PLoS ONE,
2009
, 4 (e7307).
Höltje M
et al.
Differential distribution of voltage-gated potassium channels Kv 1.1-Kv1.6 in the rat retina during development.
J. Neurosci. Res.,
2007
Jan
, 85 (19-33).
Koh SD
et al.
Contribution of delayed rectifier potassium currents to the electrical activity of murine colonic smooth muscle.
J. Physiol. (Lond.),
1999
Mar
1
, 515 ( Pt 2) (475-87).
Bertoli A
et al.
Activation and deactivation properties of rat brain K+ channels of the Shaker-related subfamily.
Eur. Biophys. J.,
1994
, 23 (379-84).
Scott VE
et al.
Antibodies specific for distinct Kv subunits unveil a heterooligomeric basis for subtypes of alpha-dendrotoxin-sensitive K+ channels in bovine brain.
Biochemistry,
1994
Feb
22
, 33 (1617-23).
Mondal S
et al.
In silico detection of binding mode of J-superfamily conotoxin pl14a with Kv1.6 channel.
In Silico Biol. (Gedrukt),
2007
, 7 (175-86).
Smart SL
et al.
Identification of the delayed rectifier potassium channel, Kv1.6, in cultured astrocytes.
Glia,
1997
Jun
, 20 (127-34).
Gómez-Hernandez JM
et al.
Molecular basis for different pore properties of potassium channels from the rat brain Kv1 gene family.
Pflugers Arch.,
1997
Nov
, 434 (661-8).
Imperial JS
et al.
A novel conotoxin inhibitor of Kv1.6 channel and nAChR subtypes defines a new superfamily of conotoxins.
Biochemistry,
2006
Jul
11
, 45 (8331-40).
Liu HL
et al.
Homology models of the tetramerization domain of six eukaryotic voltage-gated potassium channels Kv1.1-Kv1.6.
J. Biomol. Struct. Dyn.,
2005
Feb
, 22 (387-98).
Dodson PD
et al.
Two heteromeric Kv1 potassium channels differentially regulate action potential firing.
J. Neurosci.,
2002
Aug
15
, 22 (6953-61).
Glazebrook PA
et al.
Potassium channels Kv1.1, Kv1.2 and Kv1.6 influence excitability of rat visceral sensory neurons.
J. Physiol. (Lond.),
2002
Jun
1
, 541 (467-82).
Wang XC
et al.
α-dendrotoxin sensitive Kv1 channels contribute to conduction failure of polymodal nociceptive C-fibers from rat coccygeal nerve.
J. Neurophysiol.,
2015
Nov
25
, (jn.00786.2014).
Zhu J
et al.
The Kv1.3 potassium channel is localized to the cis-Golgi and Kv1.6 is localized to the endoplasmic reticulum in rat astrocytes.
FEBS J.,
2014
Aug
, 281 (3433-45).
Orts DJ
et al.
Biochemical and electrophysiological characterization of two sea anemone type 1 potassium toxins from a geographically distant population of Bunodosoma caissarum.
Mar Drugs,
2013
Mar
, 11 (655-79).
Manicam C
et al.
The Gatekeepers in the Mouse Ophthalmic Artery: Endothelium-Dependent Mechanisms of Cholinergic Vasodilation.
Sci Rep,
2016
, 6 (20322).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/6/ , accessed on 2025 Dec 08