Kv4.2
Description: potassium voltage-gated channel, Shal-related subfamily, member 2 Gene: Kcnd2 Alias: Kv4.2, kcnd2, Shal1, RK5
Kv4.2 (also known as RK5; KIAA1044; MGC119702; MGC119703), encoded by the gene KCND2, is a member of the potassium voltage-gated channel subfamily D. Kv4.2 is the main contributing current to the repolarizing phase 1 of the cardiac action potential. NCBI
Experimental data
Rat Kv4.2 gene in CHO host cells datasheet |
||
|
Click for details 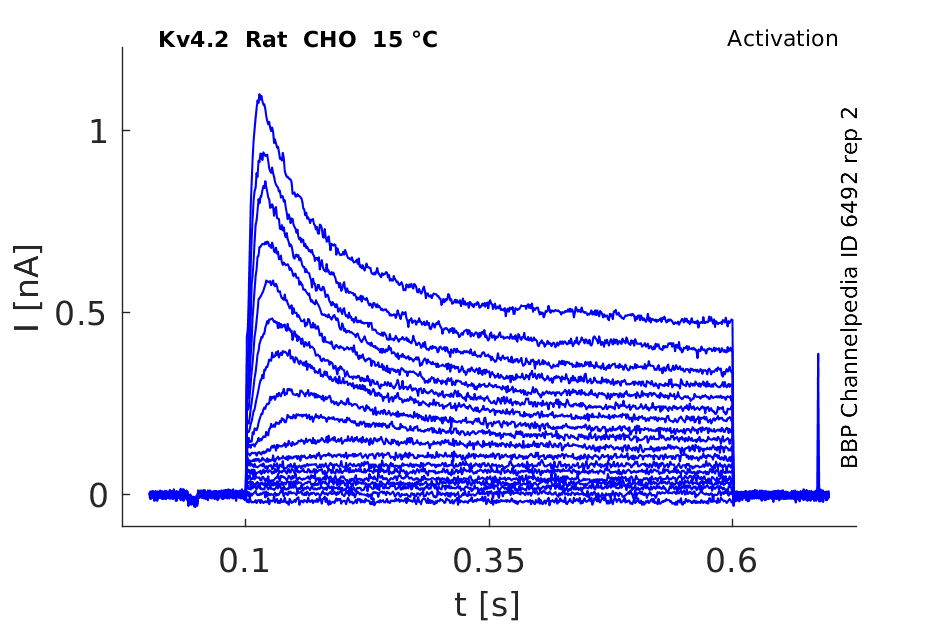
15 °Cshow 97 cells |
Click for details 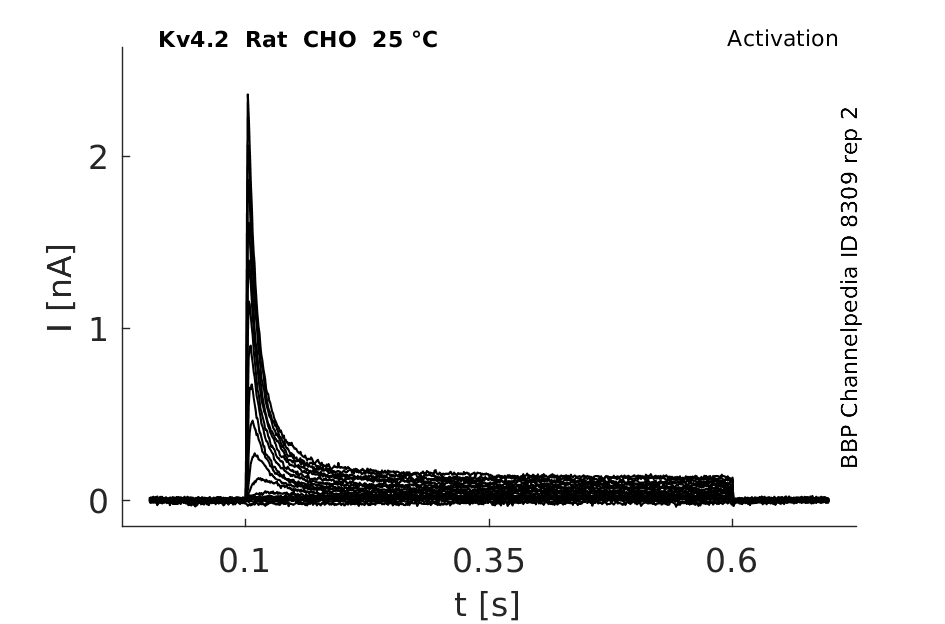
25 °Cshow 129 cells |
Click for details 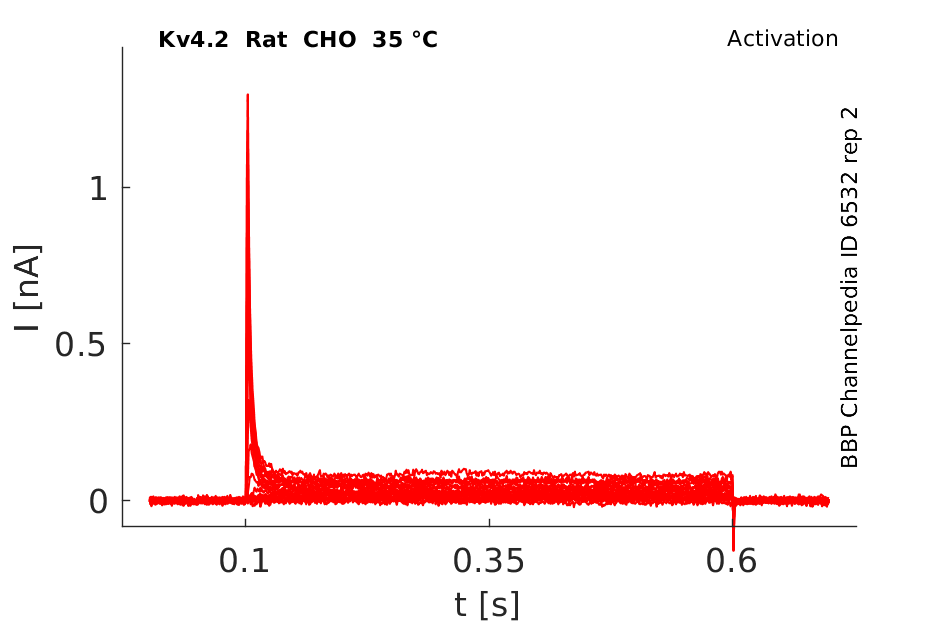
35 °Cshow 224 cells |
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_012281.3 | 5830 | |
| Mouse | NM_019697.4 | 5325 | |
| Rat | NM_031730.2 | 4018 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv4.2 Structure
Methodology for visual representation of structure available here
Crystal Structure
Tetraemic complexes of subunits
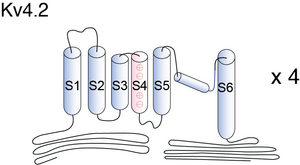
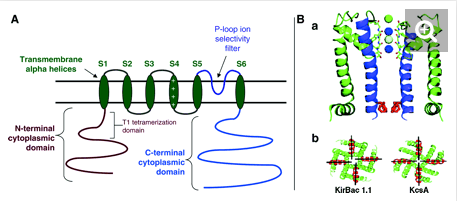
Kv4 potassium channels, like voltage-gated K+ channels, are formed from tetrameric complexes of identical or genetically related α-subunits from the same subfamily. All Kv α-subunits possess a cytoplasmic amino-terminal region, six transmembrane segments (S1–S6) plus their associated interconnecting intracellular and extracellular loops, and a cytoplasmic carboxy-terminal region. The S1–S6 region, known as the “core”, conducts the main businesses of potassium selectivity, ion conduction, and voltage-dependent gating. The S5 and S6 segments form the innermost structures and line the pore region through which K+ must traverse down its electrochemical gradient across the membrane. The S5–S6 loop, or pore (P)-loop, sits at the outer end of the “inverted teepee” formed by S5 and S6 segments from all four α-subunits and acts as the selectivity filter [467]
Kv4.2 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
Kv4.2 Kinetics
rat Kv4.2 expressed in CHO

DELETION of AMINO ACIDS ALTERS EXPERESSION
Deletions in the first 40 amino acids of the Kv4 alpha subunit N-terminus significantly increase the functional expression of Kv4.2 channels and eliminate KChIP regulation
INTERACTION WITH KCHiP
Transient transfection of the rat Kv4.2 alpha-subunit in CHO cells yielded typical A-type potassium currents. Expression of Kv4.2 together with KChIP1, 2 or 3 revealed several effects of KChIP co-expression on Kv4 currents. First, the density of Kv4.2 currents increased about 12-fold, indicating that KChIP1 may promote and/or stabilize expression of Kv4.2 at the cell surface. Second, the midpoint of voltage activation of Kv4.2 currents shifted to more hyperpolarized potentials. Furthermore, the kinetics of Kv4.2 inactivation slowed considerably, whereas KChIP/Kv4.2 channels recovered from inactivation much more rapidly versus channels produced by Kv4.2 expression alone [1195]
Kinetic Model based on Markov Model for Kv4.2

Model Kv4.2 (ID=40)
Tau is modified(multipled by 0.2) from model1
| Animal | rat | |
| CellType | Neocortical L5PC | |
| Age | 14 Days | |
| Temperature | 23.0°C | |
| Reversal | -68.7 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [296] J M Bekkers et. al; J. Physiol. (Lond.) 2000 Jun 15 | |
| mpower | 3.0 | |
| m Inf | (1/(1 + exp((v- -18.8)/-16.6))) | |
| m Tau | 1.0/((0.026* exp(-0.026*v)) + (35* exp(0.136*v))) If v lt -50 | |
| m Tau | 1.7/(1+ exp((-42 - v)/-26)) + 0.34 If v gteq -50 | |
| hpower | 1.0 | |
| h Inf | 1/(1 + exp((v - -81.6)/6.7)) | |
| h Tau | 0.01*v + 6.7 | |
Expression in mouse brain
Kv4.2 can be found in pyramidal neurons in mouse neocortex. [319]
Kv4.2 is concentrated within the perinuclear endoplasmic reticulum and Golgi compartments, with some immunoreactivity apparent at the outer margins of the cell (Fig. 3b). When KChIP1 is expressed with Kv4.2, the characteristic diffuse KChIP1 distribution changes markedly, such that KChIP1 and Kv4.2 immunofluorescence completely overlap [1195]
DISTRIBUTION IN NEURON
Kv4.2 is abundant in the dendrites of CA1 pyramidal neurons of the hippocampus.[293]. Kv4.2 and Kv4.3 are expressed in membranes of somata, dendrites, and spines of pyramidal cells and GABAergic neurons. [319]
KChIP2 co-localizes with Kv4.2 in the dendrites of granule cells in the dentate gyrus (Fig. 3d–f), in the apical and basal dendrites of hippocampal and neocortical pyramidal cells, and in several subcortical structures including the striatum and thalamus [1195]
Immunocytochemical studies have shown that the subcellular distribution of neuronal rat Kv4.2 channels is restricted to the somatodendritic area, and the high abundance of Kv4.2 in the soma and dendrites led to the hypothesis that these channels may have an important influence on postsynaptic neuronal signal transduction [1686]
Immunohistochemical analysis shows that Kv4.2 has a somatodendritic distribution, and in adult hippocampus, Kv4.2 is expressed on distal dendrites and neuropils of CA1-3 neurons. The somatodendritic membrane of rat neostriatal cholinergic interneurons express Kv4.2 but not Kv1.4 according to immunocytochemical analysis [467]
KV4.2 FUNCTION IN NEURON
In hippocampal pyramidal neurons, where the A-type channel density increases with distance from the soma, activation of Kv4.2 channels may prevent the back-propagation of action potentials. In addition, the rapid activation of A-type channels may protect the postsynaptic membrane from excessive depolarization [1687]
Inactivation of Kv4.2 channels by subthreshold EPSPs can lead to spike amplification, which provides a possible explanation for the Hebbian associativity found in distal dendrites [1688]
PROLONGING INHIBITION IN SYNAPTIC TRANSMISSION
More recently it has been shown that A-type channels localized to the dendritic spines of GABAergic interneurons in the olfactory bulb are necessary to counterbalance fast glutamatergic EPSPs, thereby allowing a prolonged inhibitory synaptic transmission in a local feedback loop. The inhibitory synaptic transmission becomes shorter and stronger when the A-type channels are inactivated by subthreshold membrane depolarization [1689]
Fragile X Syndrome, Mental Retardation
FMRP is a positive regulator of Kv4.2 mRNA translation and protein expression and associates with Kv4.2 mRNA in vivo and in vitro. Our results suggest that absence of FMRP-mediated positive control of Kv4.2 mRNA translation, protein expression, and plasma membrane levels might contribute to excess neuronal excitability in Fmr1 KO mice, and thus imply a potential mechanism underlying FXS-associated epilepsy [1827]
Neurological Disorders
Neuronal excitability is tightly regulated, and defects in mechanisms involved in this regulation can lead to neurological disorders. A key player in the control of neuronal excitability in the brain is the A-type potassium channel Kv4.2. This potassium channel controls excitatory currents in the hippocampus and is thus critical to maintain a healthy excitatory balance in the brain. Emerging data suggests that Kv4.2 protein levels are dysregulated in a variety of disease states. (http://www.ibridgenetwork.org/emory/oligonucleotide-antagonists-of-kv4-2-regulating-micrornas-mir)
Kv4.2 not involved in Neuropsychiatry
We did not find clear evidence for an involvement of Kv4.2 in neuropsychiatric or plasticity-related phenotypes, but there was support for a role in Kv4.2 in dampening excitatory responses to novel stimuli [1828]
Cardiomyopathy and Heart Failure
cardiac-specific (driven by +/-MHC promoter) overexpression of a dominant-negative Kv4.2 K+ channel subunit in mice caused dilated cardiomyopathy and heart failure, in addition to prolongation of action potential duration (APD) [1829]
High External Potassium Concentration
High external potassium concentration counteracts Shaker C-type inactivation [479] and accelerates Kv4 channel inactivation [24], [418].
KCNE3

KChIP1b and KChIP1a
The potassium channel interacting protein KChIP1b splice variant induces slow recovery from inactivation for Kv4.2 whereas KChIP1a enhanced the recovery. Reduction of the side chain bulkiness in exon1b resulted in the conversion of the KChIP1b phenotype into the KChIP1a phenotype. [31]
Calcium–calmodulin-dependent kinase II (CaMKII)
CaMKII can directly modulate neuronal excitability by increasing cell-surface expression of A-type K+ channels (Kv4.2). CaMKII phosphorylation had no effect on channel biophysical properties. [32]
DPPX (DPP6) and DPP10
Dipeptidyl aminopeptidase-like protein DPPX (DPP6) associates with Kv4 potassium channels, increasing surface trafficking and reconstituting native neuronal ISA-like properties. Coexpression of dipeptidyl peptidase 10 (DPP10) and HA-tagged DPP10 enhanced Kv4.2 current approximately fivefold without increasing protein level. [33]
Cytochalasin D
Distribution and density of Kv4.2 channels at the cell surface are primarily the result of reorganization of the actin cytoskeleton. Pretreatment of HEK cells with cytochalasin D to disrupt the actin microfilaments greatly augmented whole cell Kv4.2 currents at potentials positive to 20 mV. [34]
PrPC
Following a rapid rise to peak amplitude, the current rapidly decayed despite a continued depolarizing step command. In the presence of exogenous PrPC, the peak amplitude of the A-type K+ currents at 20 mV was larger (14.5 ± 0.9 nA; average ± S.E., n = 17) than that mediated by the Kv4.2 channel complex in its absence [1669]
KChiP1, 2 , 3
The following table emphasizes the effects of KChiPs on the Kv4 family [1195]
References
Jerng HH
et al.
Inactivation gating of Kv4 potassium channels: molecular interactions involving the inner vestibule of the pore.
J. Gen. Physiol.,
1999
May
, 113 (641-60).
Dougherty K
et al.
Gating charge immobilization in Kv4.2 channels: the basis of closed-state inactivation.
J. Gen. Physiol.,
2008
Mar
, 131 (257-73).
Barghaan J
et al.
Dynamic coupling of voltage sensor and gate involved in closed-state inactivation of kv4.2 channels.
J. Gen. Physiol.,
2009
Feb
, 133 (205-24).
Bähring R
et al.
Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels.
J. Physiol. (Lond.),
2001
Aug
15
, 535 (65-81).
Van Hoorick D
et al.
The aromatic cluster in KCHIP1b affects Kv4 inactivation gating.
J. Physiol. (Lond.),
2007
Sep
15
, 583 (959-69).
Varga AW
et al.
Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents.
J. Neurosci.,
2004
Apr
7
, 24 (3643-54).
Jerng HH
et al.
Modulation of Kv4.2 channel expression and gating by dipeptidyl peptidase 10 (DPP10).
Biophys. J.,
2004
Oct
, 87 (2380-96).
Wang Z
et al.
Increased focal Kv4.2 channel expression at the plasma membrane is the result of actin depolymerization.
Am. J. Physiol. Heart Circ. Physiol.,
2004
Feb
, 286 (H749-59).
Sanguinetti MC
et al.
Heteropodatoxins: peptides isolated from spider venom that block Kv4.2 potassium channels.
Mol. Pharmacol.,
1997
Mar
, 51 (491-8).
Kim J
et al.
Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons.
Neuron,
2007
Jun
21
, 54 (933-47).
Nerbonne JM
et al.
Electrical remodelling maintains firing properties in cortical pyramidal neurons lacking KCND2-encoded A-type K+ currents.
J. Physiol. (Lond.),
2008
Mar
15
, 586 (1565-79).
Bekkers JM
Properties of voltage-gated potassium currents in nucleated patches from large layer 5 cortical pyramidal neurons of the rat.
J. Physiol. (Lond.),
2000
Jun
15
, 525 Pt 3 (593-609).
Burkhalter A
et al.
Differential expression of I(A) channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses.
J. Neurosci.,
2006
Nov
22
, 26 (12274-82).
Serôdio P
et al.
Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain.
J. Neurophysiol.,
1998
Feb
, 79 (1081-91).
Jerng HH
et al.
Molecular physiology and modulation of somatodendritic A-type potassium channels.
Mol. Cell. Neurosci.,
2004
Dec
, 27 (343-69).
Baukrowitz T
et al.
Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms.
Neuron,
1995
Oct
, 15 (951-60).
Kaulin YA
et al.
Mechanism of the modulation of Kv4:KChIP-1 channels by external K+.
Biophys. J.,
2008
Feb
15
, 94 (1241-51).
An WF
et al.
Modulation of A-type potassium channels by a family of calcium sensors.
Nature,
2000
Feb
3
, 403 (553-6).
Bähring R
et al.
Conserved Kv4 N-terminal domain critical for effects of Kv channel-interacting protein 2.2 on channel expression and gating.
J. Biol. Chem.,
2001
Jun
29
, 276 (23888-94).
Mercer RC
et al.
The prion protein modulates A-type K+ currents mediated by Kv4.2 complexes through dipeptidyl aminopeptidase-like protein 6.
J. Biol. Chem.,
2013
Dec
27
, 288 (37241-55).
Wang W
et al.
Functional Significance of K+ Channel β-Subunit KCNE3 in Auditory Neurons.
J. Biol. Chem.,
2014
Apr
11
, ().
Sheng M
et al.
Subcellular segregation of two A-type K+ channel proteins in rat central neurons.
Neuron,
1992
Aug
, 9 (271-84).
Hoffman DA
et al.
K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons.
Nature,
1997
Jun
26
, 387 (869-75).
Magee JC
et al.
A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons.
Science,
1997
Jan
10
, 275 (209-13).
Schoppa NE
et al.
Regulation of synaptic timing in the olfactory bulb by an A-type potassium current.
Nat. Neurosci.,
1999
Dec
, 2 (1106-13).
Gross C
et al.
Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2.
J. Neurosci.,
2011
Apr
13
, 31 (5693-8).
Kiselycznyk C
et al.
Effects of genetic deletion of the Kv4.2 voltage-gated potassium channel on murine anxiety-, fear- and stress-related behaviors.
Biol Mood Anxiety Disord,
2012
, 2 (5).
Guo W
et al.
Molecular basis of transient outward K+ current diversity in mouse ventricular myocytes.
J. Physiol. (Lond.),
1999
Dec
15
, 521 Pt 3 (587-99).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/16/ , accessed on 2026 Mar 12