Kv3.4
Description: potassium voltage gated channel, Shaw-related subfamily, member 4 Gene: Kcnc4 Alias: Kv3.4, kcnc4, Kcr2-4, KSHIIIC
Kv3.4, encoded by the gene KCNC4, is a voltage-gated potassium channel. Kv3.4 plays a key role in responding to chronic hypoxia, linked to Alzheimer's and Parkinson's diseases, influences resting potential in skeletal muscle, acts as a modulator in neuron channels, and plays a role in cell cycle progression in uterine smooth muscle cells.
Experimental data
Rat Kv3.4 gene in CHO host cells datasheet |
||
|
Click for details 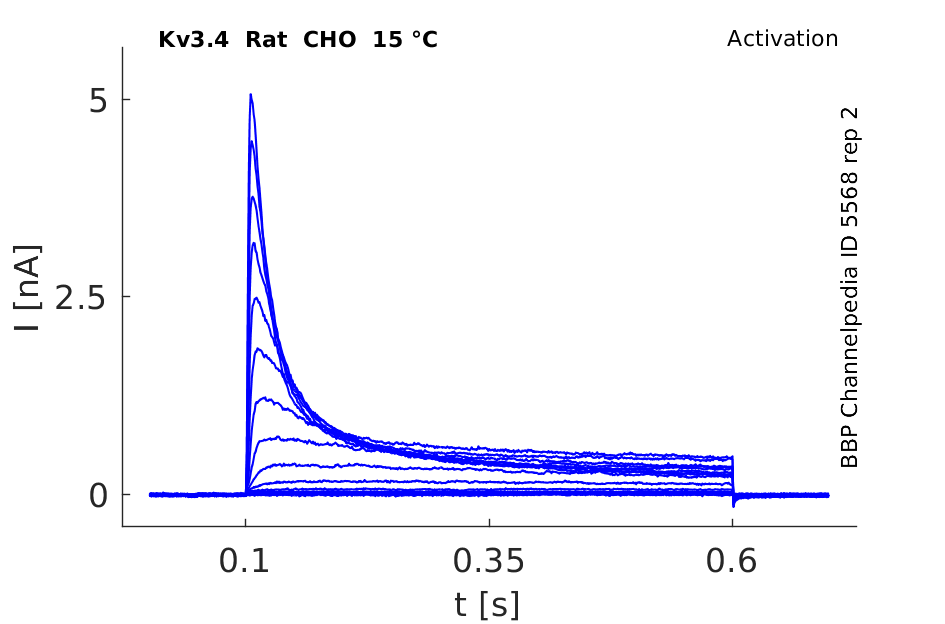
15 °Cshow 49 cells |
Click for details 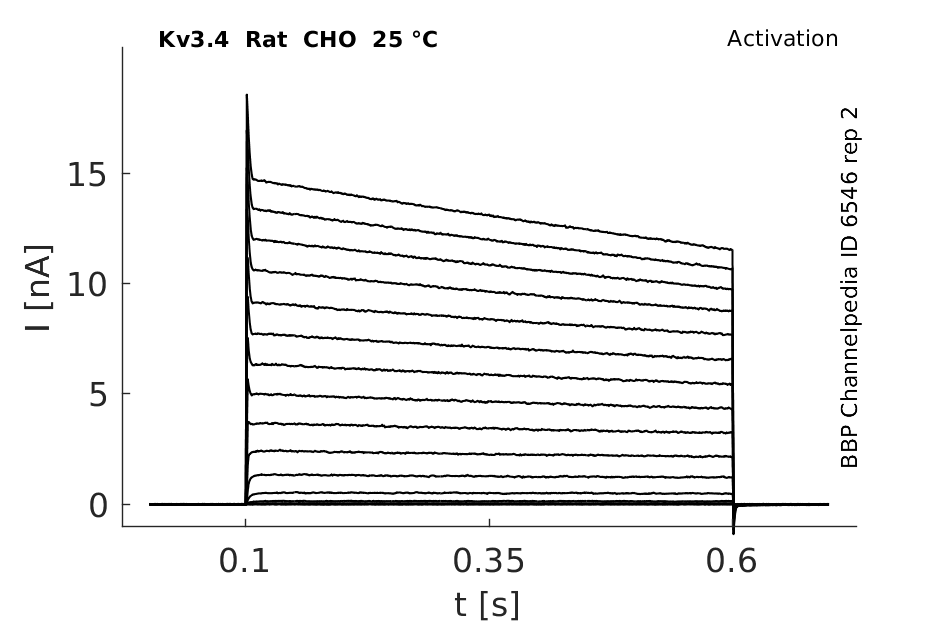
25 °Cshow 896 cells |
Click for details 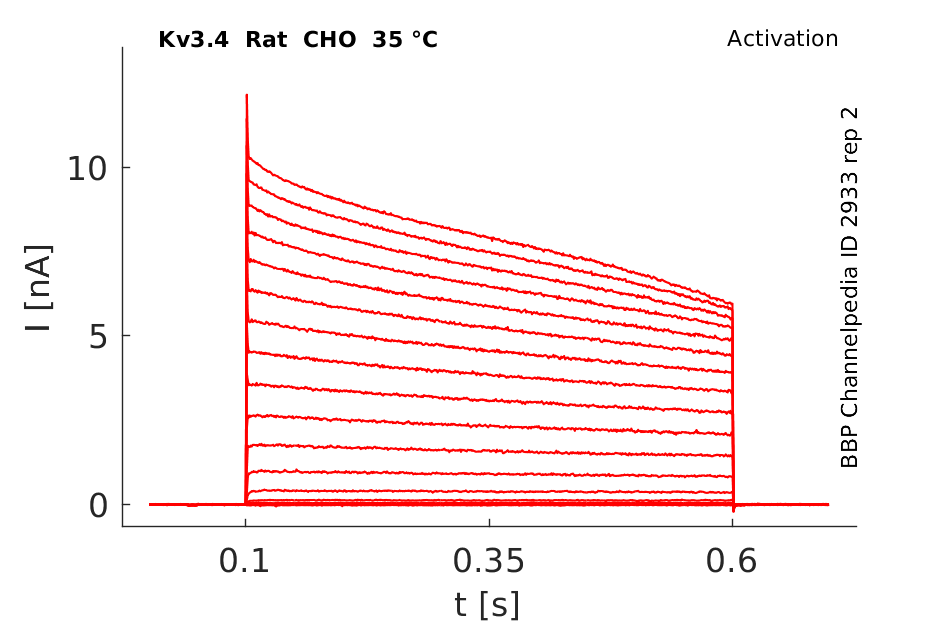
35 °Cshow 84 cells |
Mouse Kv3.4 gene in CHO host cells |
||
|
Click for details 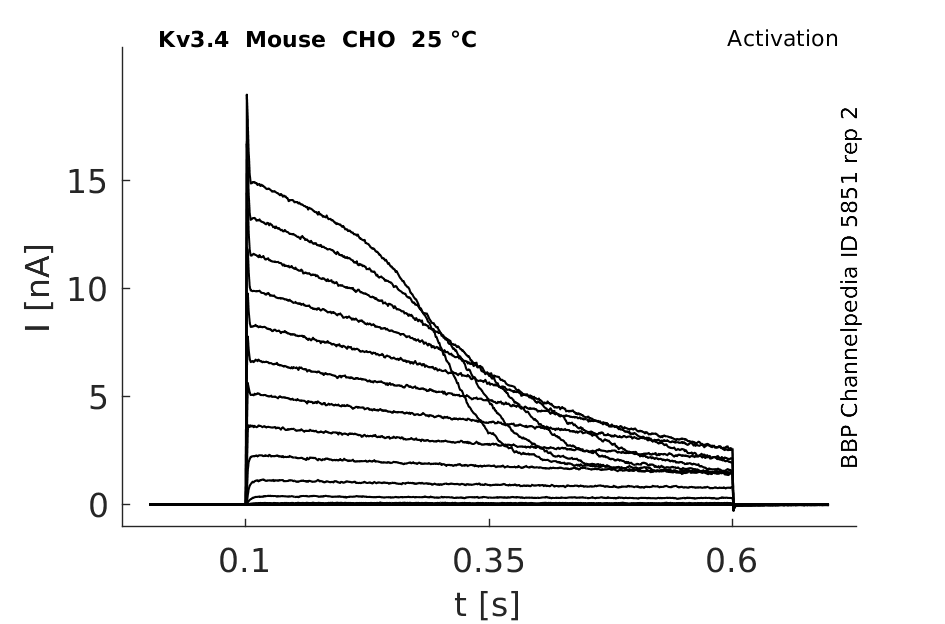
25 °Cshow 307 cells |
||
Human Kv3.4 gene in CHO host cells |
||
|
Click for details 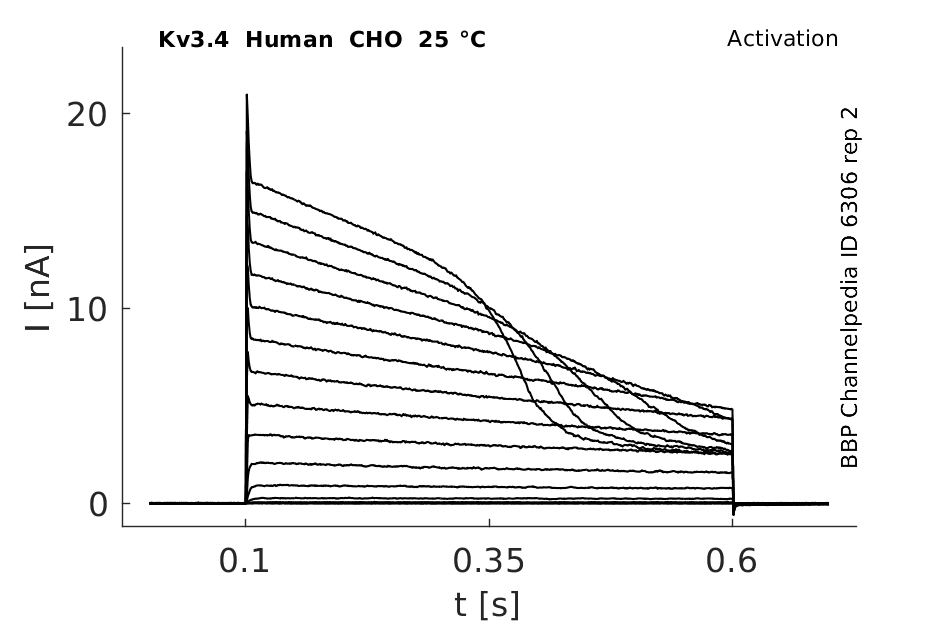
25 °Cshow 65 cells |
||
Rat Kv3.4 gene in HEK host cells |
||
|
Click for details 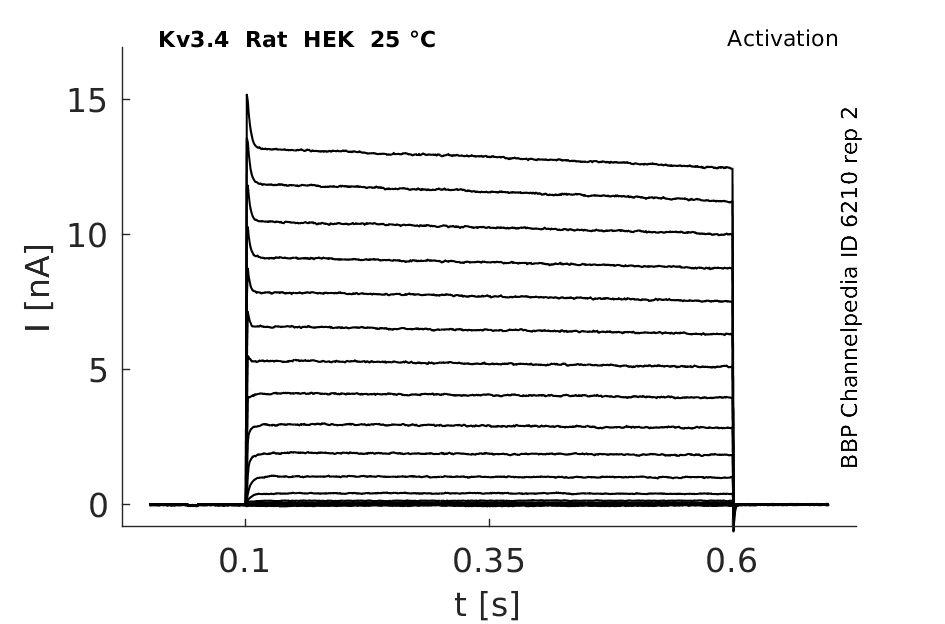
25 °Cshow 194 cells |
||
Rat Kv3.4 gene in CV1 host cells |
||
|
Click for details 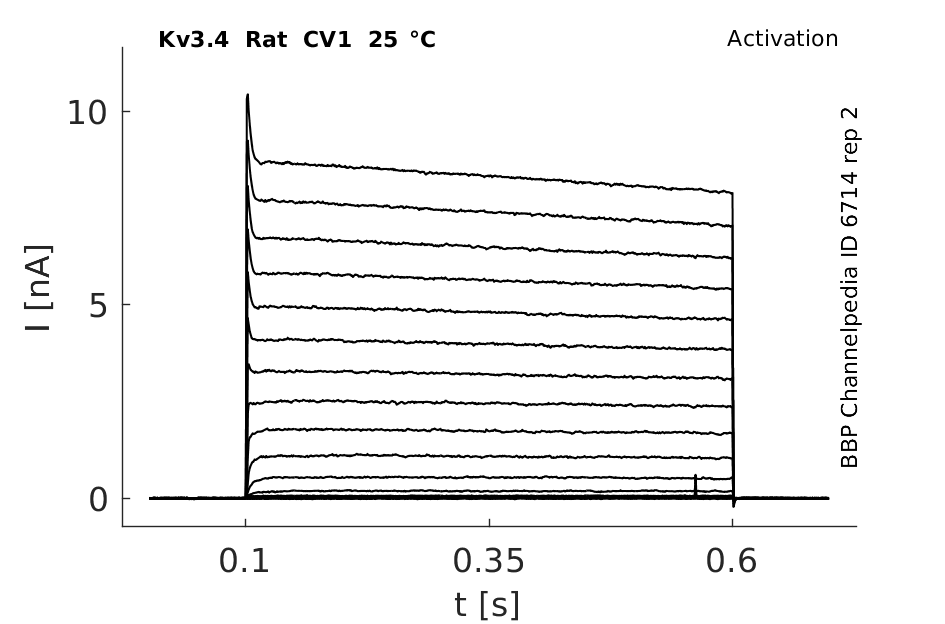
25 °Cshow 141 cells |
||
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_004978.6 | 4829 | |
| Mouse | NM_001356447.1 | 2953 | |
| Rat | NM_001415110.1 | 4318 |
Protein Isoforms
Isoforms
Post-Translational Modifications
Visual Representation of Kv3.4 Structure
Methodology for visual representation of structure available here
KV3.4
Structure of inactivation gates derived from the mammalian Kv channel 3.4. Backbone (left) and surface structure (right). Backbone representation shows 8th lowest-energy structures for each peptide. N and C, NH2 and COOH terminals, respectively. Kv3.4 peptide showed compact folding through- out the molecule but did not exhibit any typical secondary structure
Kv3.4 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
A crucial property of the currents mediated by Kv3.1 and Kv3.2 channels (as well as Kv3.3 and Kv3.4 channels before they fully inactivate) is their fast rate of deactivation upon repolarization (e.g. at −70 mV, Kv3.1b currents deactivate with a time constant of ≤1 ms at room temperature), which is significantly faster than that of any other known neuronal voltage-gated K+ channels by about an order of magnitude. [496]
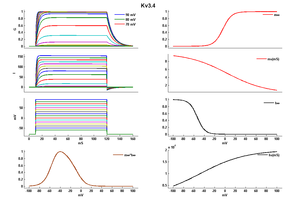
Kv3.4 Kinetics
In Xenopus laevis oocytes, hKv3.4 expresses avoltage- dependent outward K+current which, in response to a step depolarization, inactivates rapidly and almost completely within approximately 100 ms. Whole-oocyte outward K+ currents were elicited by 112 ms step depolarizations between -50 and +50 mV in 10 mV increments from a holding potential of -100 mV [1642]
Single Channel Currents

Rat Kv3.4 Kinetics Expressed in CHO Cells

Compare wit figure 1 in [302].
Model Kv3.4 (ID=29)
| Animal | Xenopus | |
| CellType | oocyte | |
| Age | 0 Days | |
| Temperature | 23.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [281] H Moreno et. al; Proc. Biol. Sci. 1992 Apr 22 | |
| mpower | 1.0 | |
| m Inf | 1/(1+exp(((v -(-3.400))/(-8.400)))) | |
| m Tau | 10.000/(1+exp(((v -(4.440))/(38.140)))) | |
| hpower | 1.0 | |
| h Inf | 1/(1+exp(((v -(-53.320))/(7.400)))) | |
| h Tau | 20000.000/(1+exp(((v -(-46.560))/(-44.140)))) | |
Kv3.4 transcripts are abundant in skeletal muscle and sympathetic neurons, but are only weakly expressed in a few neuronal types in the brain, often in neurons that also express other Kv3 genes. [290]
Kv3.4 channel subunits can be found throughout the thoracic spinal cord and brainstem, including regions involved in autonomic control.
Expression in Rat Brain
Transcripts of three of these genes,KV3.1, KV3.3, and KV3.4, exhibit localizations more similar to eachother than thoseof KV3.2 transcripts. At thesametime, many regions that express KV3.1, KV3.3, or KV3.4 mRNAs prominently, such as the cerebellar cortex, the spinalcord, the reticular thalamic nucleus, the inferior colliculus, and many nuclei in the brainstem, appear to express little or no KV3.2 transcripts [1644]
Ultrastructural analysis also revealed Kv3.4-IR postsynaptically in somata and dendrites, where it was often clustered at postsynaptic densities.
The boxed areas in A are shown at higher magnification in B–D. Kv3.4-IR is present in both the presynaptic terminals and in the postsynaptic dendrite, where it appeared to be located close to the active zone, indicating a possible relationship with the PSD [1643]
Kv3.4 subunits have been implicated recently in responses to chronic hypoxia [492] and, significantly, in the etiology of Alzheimer’s [493] and Parkinson’s diseases [494].
Kv3.4 subunits associate with MiRP2 proteins in skeletal muscle to form subthreshold-operating channels that contribute to setting the resting potential of muscle cells. [497]
Neuronal Function
Kv3.4 is expressed in neurons also expressing KV3.1 or KV3.3 mRNAs, KV3.4 subunits may act in CNS neurons as modulators of the inactivation properties of channels composed mainly of KV3.1 and KV3.3 proteins. The electrophysiological studies described in this article indicate that small amounts of KV3.4 transcripts might be sufficient to impart fast inactivating properties to channels composed mainly of the other ShIII sub- units. Similar studies with ShI subunits have also shown that the presence of a single inactivating subunit is sufficient to impart inactivating properties in the resultant channels [1644]
Uterine Artery Smooth Muscle Cells
Kv3.4 channels exert a permissive role in the cell cycle progression of proliferating uterine VSMCs, as their blockade induces cell cycle arrest after G2/M phase completion. The modulation of resting membrane potential (V(M)) by Kv3.4 channels in proliferating VSMCs suggests that their role in cell cycle progression could be at least in part mediated by their contribution to the hyperpolarizing signal needed to progress through the G1 phase [1750]
BDS-I and BDS-II
Sea anemone toxins BDS-I and BDS-II (“blood depressing substance”) are highly specific blockers for Kv3.4 subunits in expression systems.[495]
BDS is not subunit selective but also inhibits Kv3.1 and Kv3.2 subunits.[23]
PMA
Phorbol 12-myristate 13-acetate (20nM)reduced inactivation of the current [1642]
OAG
1-oleoyl-2-acetyl-rac-glycerol (60uM) also reduced inactivation of the current in a time dependent manner [1642]
Serine 82 of MiRP2
Phosphorylation of serine 82 of MiRP2 (MiRP2-S82P) is required for MiRP2 to produce the normal shift in voltagedependent activation of Kv3.4 observed in vivo. [21]
1,4-diazabicyclo[2.2.2]octane (DABCO)
The bicyclic molecule 1,4-diazabicyclo[2.2.2]octane (DABCO) does not inhibit Kv2.1 or Kv3.4 (1 mM), but submillimolar concentrations of various cell-safe substituted mono- and di-DABCO forms inhibit both channels whereas Kv4.2 channels are relatively insensitive. [22]
Kv3.1
Kv3.4 subunit coassembles with Kv3.1 subunits in rat brain FS neurons. Coassembly enhances the spike repolarizing efficiency of the channels, thereby reducing spike duration and enabling higher repetitive spike rates. These results suggest that manipulation of K3.4 subunit expression could be a useful means of controlling the dynamic range of FS neurons [1633]
Cav1.2
Kv3.4 and Cav1.2, two high-voltage-activated ion channels, may act together to control Ca²⁺ dependent electrical activity of pioneer axons and play important roles during axon pathfinding [1646]
Alcohol
a hydrophobic point mutation within a cytoplasmic loop of an ethanol-insensitive K+ channel (human Kv3.4) was sufficient to allow significant inhibition by n-alkanols, with a dose-inhibition relation that closely resembled that of wildtype Shaw2 channels [1749]
References
Abbott GW
et al.
Phosphorylation and protonation of neighboring MiRP2 sites: function and pathophysiology of MiRP2-Kv3.4 potassium channels in periodic paralysis.
FASEB J.,
2006
Feb
, 20 (293-301).
Gordon E
et al.
1,4-Diazabicyclo[2.2.2]octane derivatives: a novel class of voltage-gated potassium channel blockers.
Mol. Pharmacol., 2006 Mar , 69 (718-26).
Yeung SY
et al.
Modulation of Kv3 subfamily potassium currents by the sea anemone toxin BDS: significance for CNS and biophysical studies.
J. Neurosci.,
2005
Sep
21
, 25 (8735-45).
Vega-Saenz de Miera E
et al.
Cloning of ShIII (Shaw-like) cDNAs encoding a novel high-voltage-activating, TEA-sensitive, type-A K+ channel.
Proc. Biol. Sci.,
1992
Apr
22
, 248 (9-18).
Rudy B
et al.
Contributions of Kv3 channels to neuronal excitability.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (304-43).
Rudy B
et al.
Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing.
Trends Neurosci.,
2001
Sep
, 24 (517-26).
Prüss H
et al.
Age-dependent axonal expression of potassium channel proteins during development in mouse hippocampus.
,
2009
Dec
12
, ().
Brew HM
et al.
Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse.
J. Neurosci.,
1995
Dec
, 15 (8011-22).
Martina M
et al.
Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus.
J. Neurosci.,
1998
Oct
15
, 18 (8111-25).
Kääb S
et al.
Down regulation of Kv3.4 channels by chronic hypoxia increases acute oxygen sensitivity in rabbit carotid body.
J. Physiol. (Lond.),
2005
Jul
15
, 566 (395-408).
Angulo E
et al.
Up-regulation of the Kv3.4 potassium channel subunit in early stages of Alzheimer's disease.
J. Neurochem.,
2004
Nov
, 91 (547-57).
Baranauskas G
et al.
Delayed rectifier currents in rat globus pallidus neurons are attributable to Kv2.1 and Kv3.1/3.2 K(+) channels.
J. Neurosci.,
1999
Aug
1
, 19 (6394-404).
Diochot S
et al.
Sea anemone peptides with a specific blocking activity against the fast inactivating potassium channel Kv3.4.
J. Biol. Chem.,
1998
Mar
20
, 273 (6744-9).
Coetzee WA
et al.
Molecular diversity of K+ channels.
Ann. N. Y. Acad. Sci.,
1999
Apr
30
, 868 (233-85).
Abbott GW
et al.
MiRP2 forms potassium channels in skeletal muscle with Kv3.4 and is associated with periodic paralysis.
Cell,
2001
Jan
26
, 104 (217-31).
Baranauskas G
et al.
Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons.
Nat. Neurosci.,
2003
Mar
, 6 (258-66).
Covarrubias M
et al.
Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate.
Neuron,
1994
Dec
, 13 (1403-12).
Brooke RE
et al.
Association of potassium channel Kv3.4 subunits with pre- and post-synaptic structures in brainstem and spinal cord.
Neuroscience,
2004
, 126 (1001-10).
Weiser M
et al.
Differential expression of Shaw-related K+ channels in the rat central nervous system.
J. Neurosci.,
1994
Mar
, 14 (949-72).
Iverson LE
et al.
The role of the divergent amino and carboxyl domains on the inactivation properties of potassium channels derived from the Shaker gene of Drosophila.
J. Neurosci.,
1990
Sep
, 10 (2903-16).
Huang CY
et al.
Co-expression of high-voltage-activated ion channels Kv3.4 and Cav1.2 in pioneer axons during pathfinding in the developing rat forebrain.
,
2012
Apr
2
, ().
Antz C
et al.
Fast Inactivation of Voltage-Gated K(+) Channels: From Cartoon to Structure.
News Physiol. Sci.,
1998
Aug
, 13 (177-182).
Covarrubias M
et al.
Alcohols inhibit a cloned potassium channel at a discrete saturable site. Insights into the molecular basis of general anesthesia.
J. Biol. Chem.,
1995
Aug
18
, 270 (19408-16).
Miguel-Velado E
et al.
Cell cycle-dependent expression of Kv3.4 channels modulates proliferation of human uterine artery smooth muscle cells.
Cardiovasc. Res.,
2010
Jun
1
, 86 (383-91).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/14/ , accessed on 2026 Feb 07