Kv1.5
Description: potassium voltage-gated channel, shaker-related subfamily, member 5 Gene: Kcna5 Alias: Kv1.5, kcna5
Kv1.5, encoded by the gene KCNA5, is a member of the potassium voltage-gated channel subfamily A. Kv1.5 is expressed in many other organs, such as pulmonary arteries, brain, skeletal muscle, and have crucial function in cell cycle regulation. Kv1.5 underlies the cardiac ultra-rapid delayed rectifier potassium current (IKur) in humans. IKur has important roles in controlling action potential (AP) shape and contractility in the human atrium [1573]
Experimental data
Rat Kv1.5 gene in CHO host cells datasheet |
||
|
Click for details 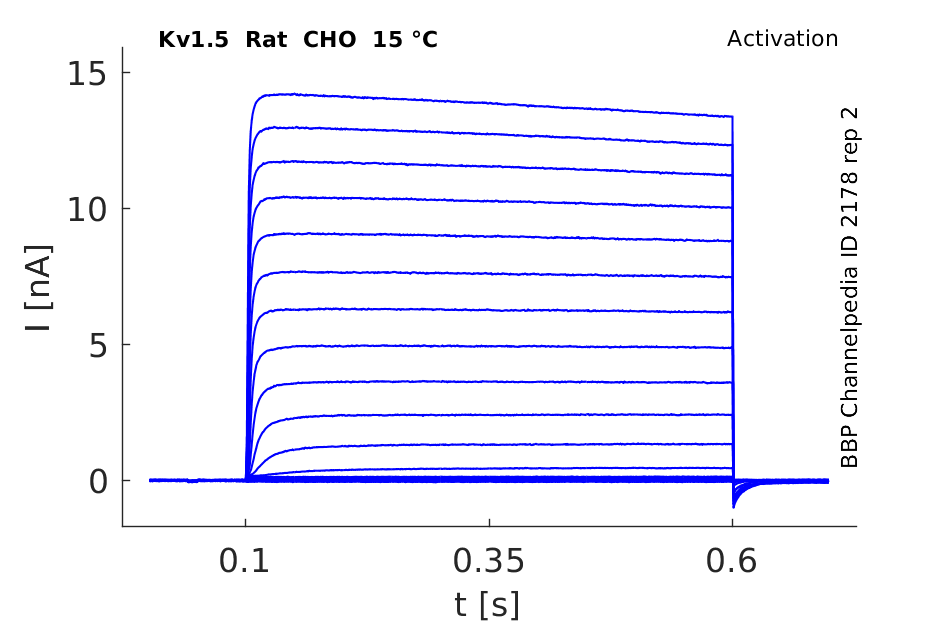
15 °Cshow 58 cells |
Click for details 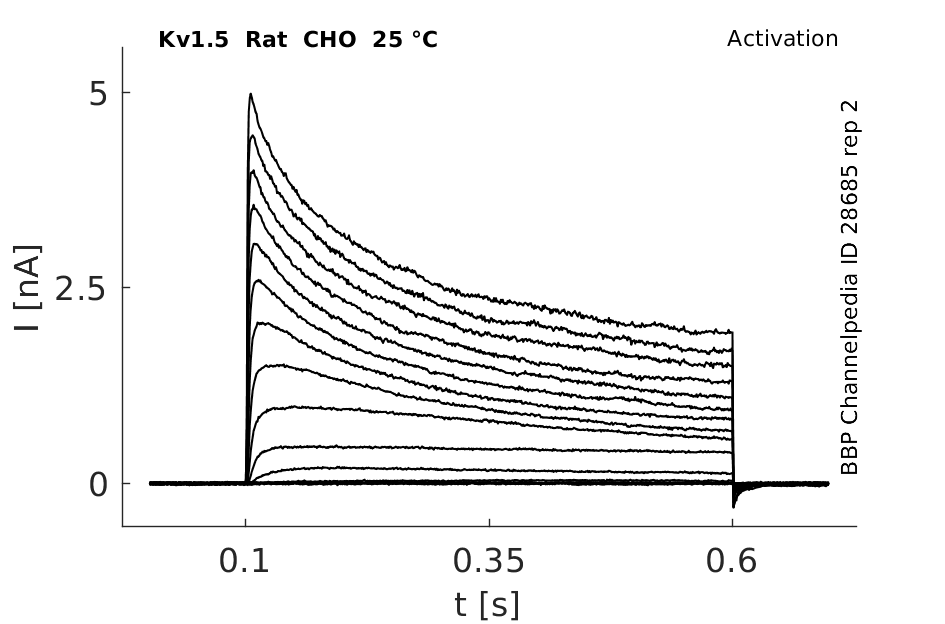
25 °Cshow 226 cells |
Click for details 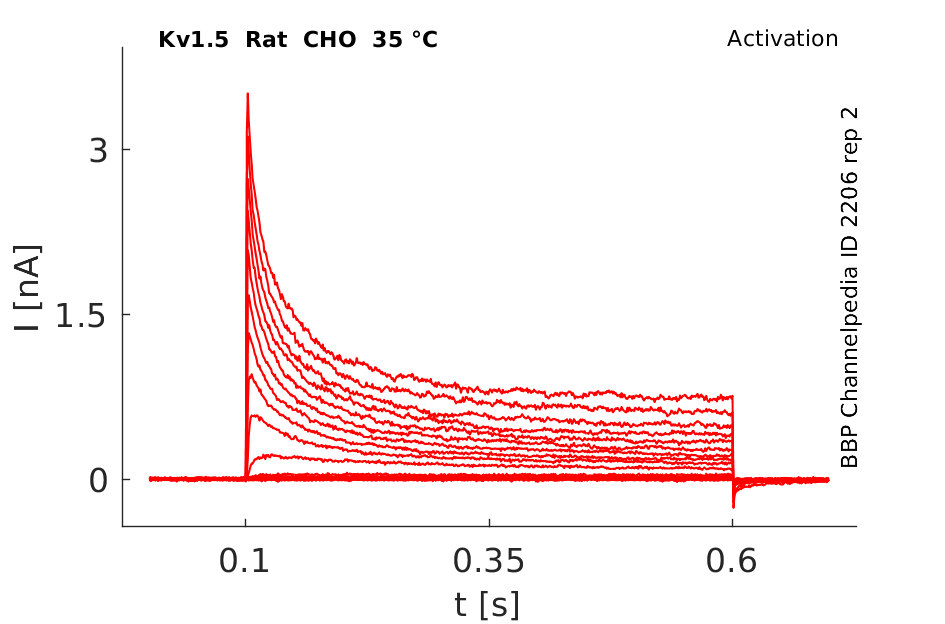
35 °Cshow 72 cells |
Mouse Kv1.5 gene in CHO host cells |
||
|
Click for details 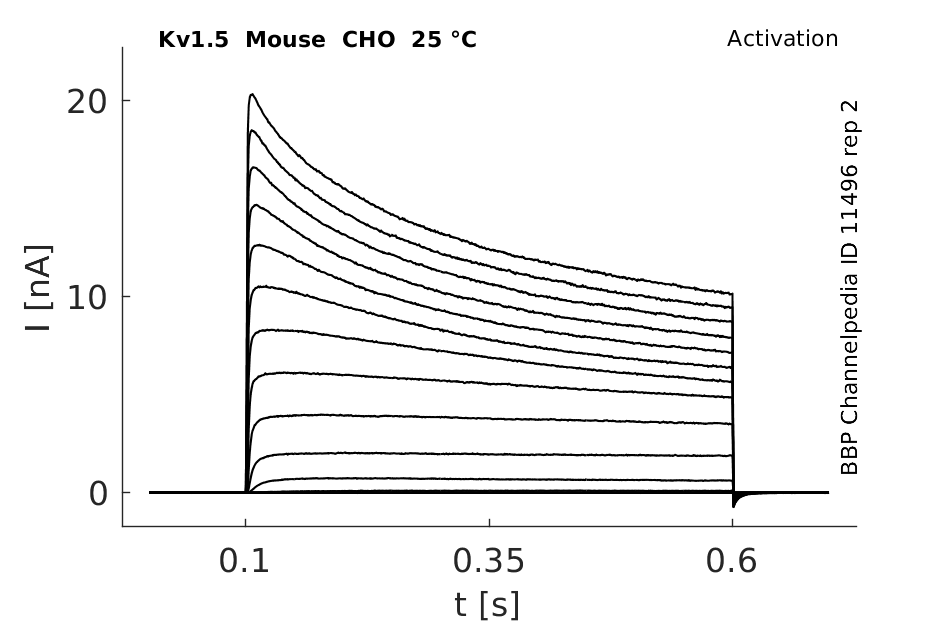
25 °Cshow 64 cells |
Click for details 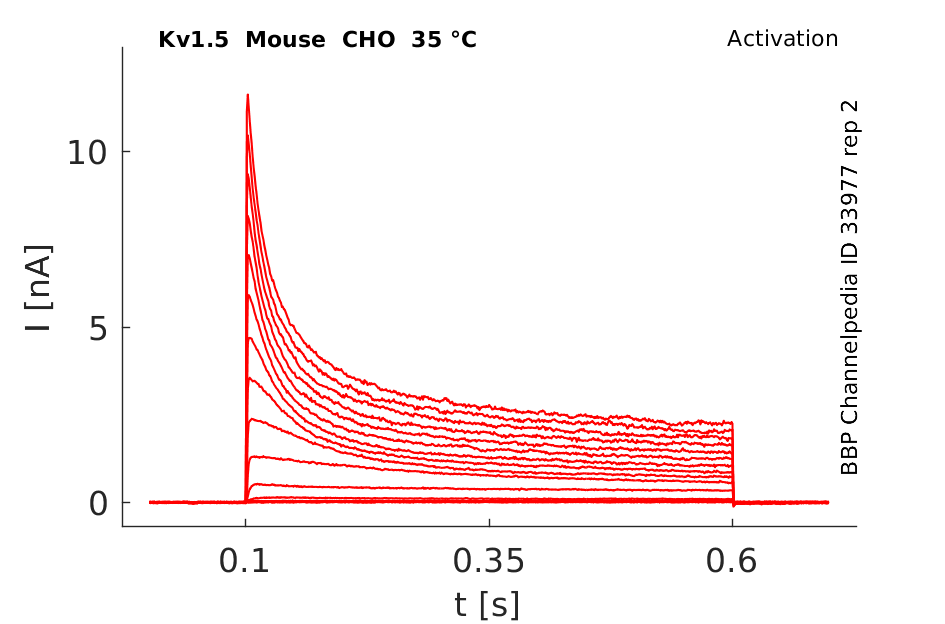
35 °Cshow 26 cells |
|
Human Kv1.5 gene in CHO host cells |
||
|
Click for details 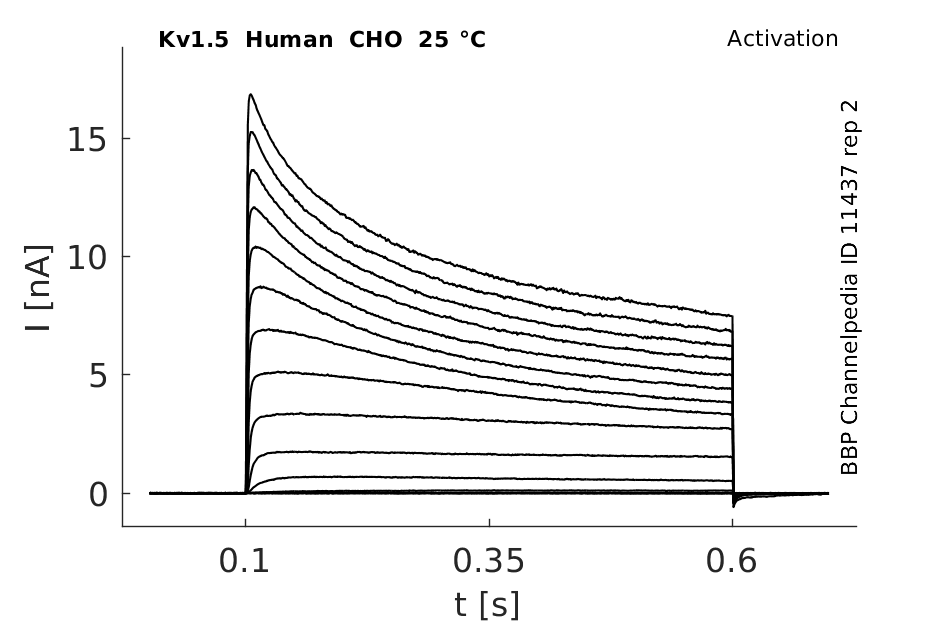
25 °Cshow 56 cells |
Click for details 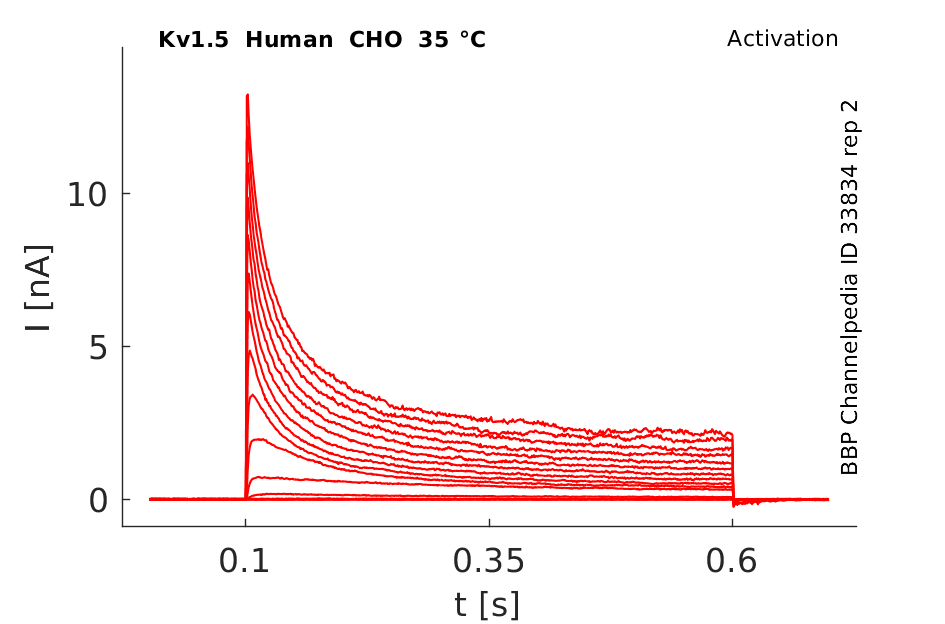
35 °Cshow 70 cells |
|
Rat Kv1.5 gene in HEK host cells |
||
|
Click for details 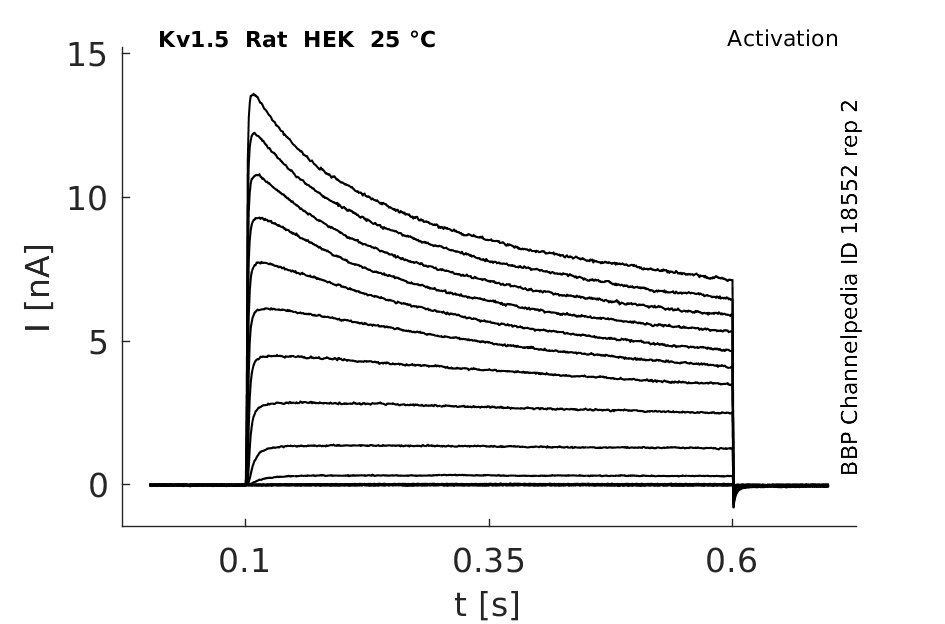
25 °Cshow 152 cells |
Click for details 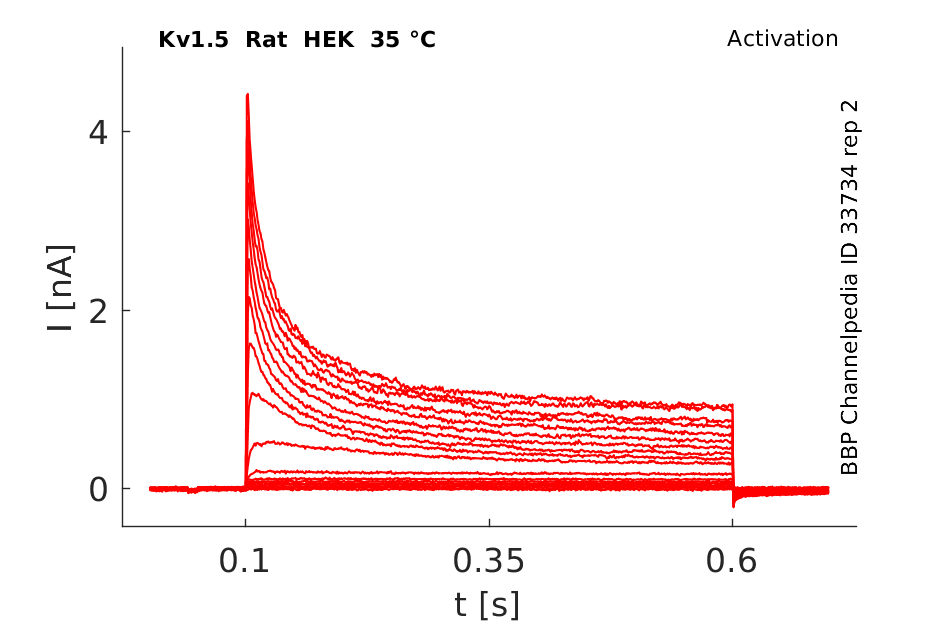
35 °Cshow 27 cells |
|
Rat Kv1.5 gene in CV1 host cells |
||
|
Click for details 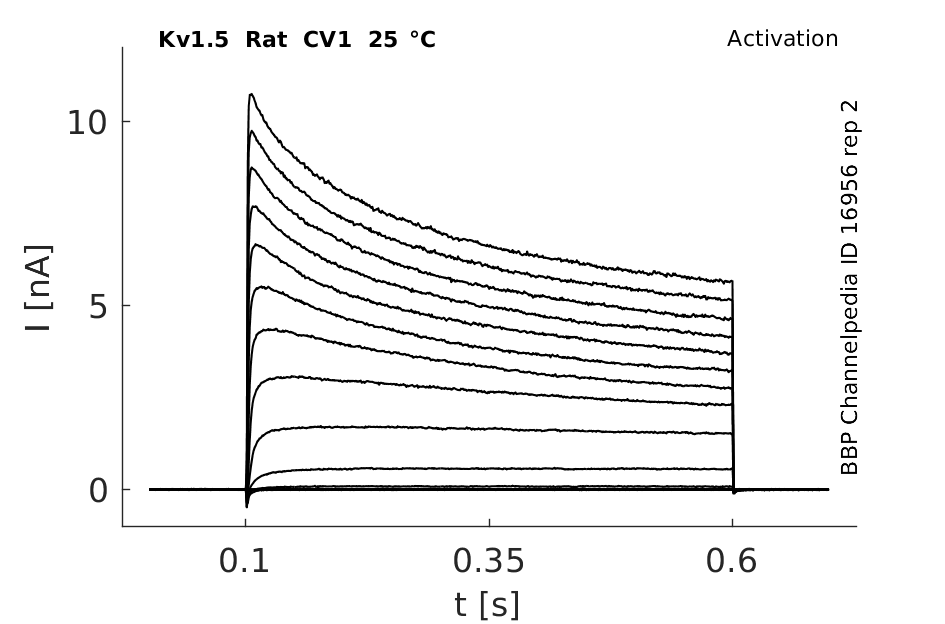
25 °Cshow 124 cells |
Click for details 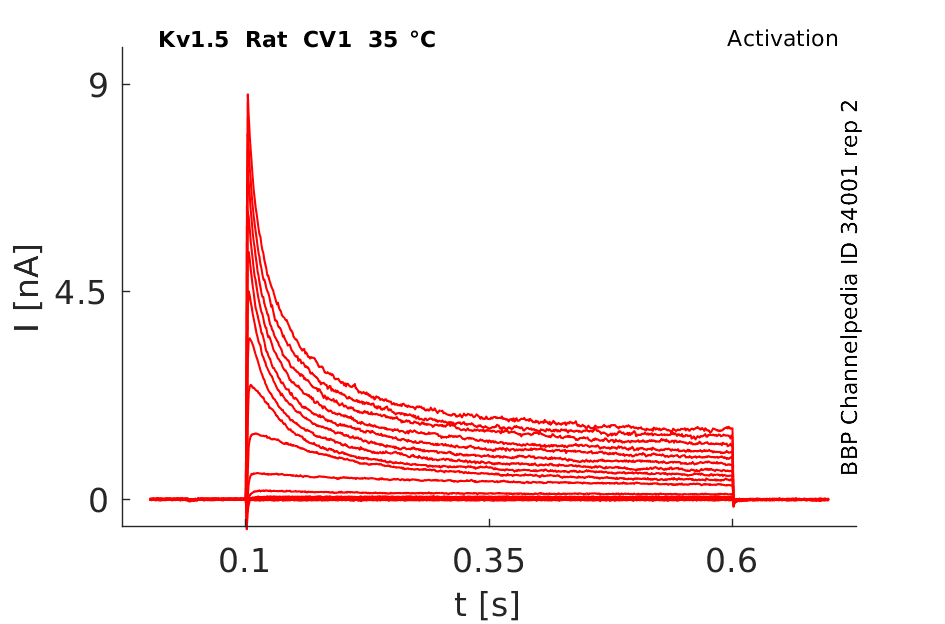
35 °Cshow 97 cells |
|
Gene
Transcript
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_002234.4 | 2910 | |
| Mouse | NM_145983.2 | 3032 | |
| Rat | NM_012972.1 | 1809 |
Isoforms
Post-Translational Modifications
Visual Representation of Kv1.5 Structure
Methodology for visual representation of structure available here
Crystal structure
The homology model of Kv1.5 is structurally more or less identical to the template, i.e., the pore region of the crystal structure of Kv1.2 (PDB accession code 2A79), which is not surprising, given the very high sequence identity in this region [1576]
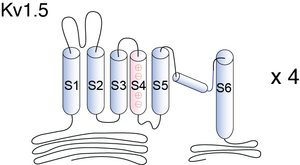
Each Kv1.5 α-subunit consists of six transmembrane spanning segments (S1–S6) with intracellular amino- and carboxy-termini. The extracellular linker between S5 and S6 dips back into the membrane. Together with the linkers from the other three α-subunits, this P-region lines the centrally located hydrophilic pore that is responsible for ion selectivity and permeation [1574]
The beta-subunits of the Kv channels (Kvb1–4) are cytosolic proteins that bind to the cytoplasmic T1 domain of the membrane spanning Kv1 and Kv4 proteins [589].
Kv1.5 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
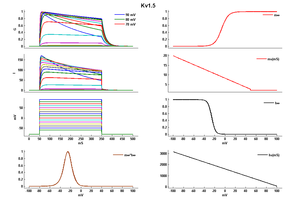
Effect of Temperature on Kinetics
Reduced-temperature (even at 34C) culture substantially enhanced the hKv1.5 currents, which is associated with stabilization of the channel protein, slower degradation of its immature protein and the accumulation of mature protein on the plasma membrane [1573]
Though the Kv1.5 current can be regarded as a ‘sustained’ current at room temperature, inactivation proceeds much faster at more physiological temperature. Moreover, the time course of recovery from inactivation is also faster at higher temperatures [1574]

Single Channel Current
the single channel Kvl.5 currents recorded in the cell attached mode.The cell was bathed with the K+-rich solution (140mM K+),and the pipette contained the external medium(4mM K+).The linear slope conductance was 8pS. Single-channel currents illustrated here were recorded at+60mV [1578]
Kv1.5 Expressed in CHO cells

Kv1.5 currents in Xenopus oocytes and effect of corticoids
Kv1.5 currents were recorded using the two-microelectrode voltage clamp technique. A series of voltage pulses of 2s were applied from −80 mV to +50 mV in 10mV steps from a holding potential of −100 mV. Application of cortisone or hydrocortisone inhibited peak and steady-state currents.[2032]
Kv1.5 currents in Xenopus oocytes
Kv1.5 currents were measured in Xenopus oocytes using the double-electrode voltage-clamp technique. Using 10mV voltage steps of -90 to 50 mV from a holding potential of – 80 mV, Kv1.5 channels elicited rapidly activating currrents and slow inactivating currents.[2031]
hKv1.5 currents recorded in CHO cells
Membrane currents of hKv1.5 channels expressed in CHO cells were recorded with the whole-cell patch-clamp technique. A series of test potentials from −50 to +50 mV lasting 300-ms in 10 mV steps were applied from a holding potential of −80 mV and a return potential of −40 mV. Fast activation of hKv1.5 currents was elicited at potentials above −20 mV.[2037]
Kv1.5 currents and inactivation kinetics in CHO cells
Traces of Kv1.5 currents were recorded by whole-cell patch-clamp technique in CHO cells. Depolarizing pulses of 250ms from -80 to +50 mV were applied that elicited rapid activation and very slow inactivation. Inactivation kinetics were recorded after a 250ms depolarizing pulse of +50ms and by applying a 250-ms repolarizing pulse at -40 mV, using a holding potential of -80 mV. The fast decline of the tail current had a time constant of 16.2±2.0 ms.[2041]
Kv1.5 activation and inactivation kinetics in HEK cells
KCNA5 was expressed in HEK cells. After 200ms pre-pulses from -50 to +50 mV, Kv1.5 peak currents were quantified during a -60 mV test-pulse. A two-pulse protocol was used to study Kv1.5 inactivation kinetics with test pulses to +70 mV and 200 ms that were applied after a 1 s pre-pulse of -60 to +40 mV.[2042]
Five State Kv1.5 markov model
Under the assumption that transition rates between the open and closed conformations of a subunit are independent of the state of the other subunits and that all four subunits operate in an identical manner, the Kv1.5 channel can be represented by the five-state model in Fig. 1A. It has four closed, nonconducting states, C1-C1, and an open, conducting state, O. Here, α and β are the forward and reverse rate constants of the transition to the open conformation, respectively [426]
Kv1.5 blockers are selectively blocking only the activated or open state of Kv1.5 channels and important features of the drug-ion channel interaction such as use- and voltage-dependence are not easily implemented in the Hodgkin-Huxley[426]
Model Kv1.5 (ID=21)
Original KSlow
| Animal | Human | |
| CellType | Oocyte | |
| Age | 0 Days | |
| Temperature | 9.0°C | |
| Reversal | -90.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [275] L H Philipson et. al; Proc. Natl. Acad. Sci. U.S.A. 1991 Jan 1 | |
| mpower | 1.0 | |
| m Inf | 1.0000/(1+ exp((v - -6.0000)/-6.4000)) | |
| m Tau | (-0.1163 * v) + 8.3300 If v lt 50 | |
| m Tau | 2 If v gteq 50 | |
| hpower | 1.0 | |
| h Inf | 1.0000/(1+ exp((v - -25.3000)/3.5000)) | |
| h Tau | (-15.5000 * v) + 1620.0000 If v lt 100 | |
| h Tau | 50 If v gteq 100 | |
Expression in Human Tissue
Kv1.5 channels are also expressed in many other organs, such as pulmonary arteries, brain, skeletal muscle [1574]
Expression of Kv1.5 in Heart
The voltage-gated K+ channel Kv1.5 is expressed in the human heart, including the atria [582], [583] and ventricles [582], brain [582], macrophages [584], as well as vascular [585], intesti- nal, and airway [586] smooth muscle. [123].
Expression of Kv1.5 in Rat
Northern blot analysis revealed that in rat Kv1.5 mRNA is expressed in many different tissues, for example, heart, kidney, skeletal muscle, lung, brain, pituitary and aorta. In cardiac tissue Kv1.5 is much more abundantly expressed in atrium than in ventricle [1585]
Interestingly, although Kv1.5 was abundantly found in the central nervous system, it stained no peripheral nerves (PNS) [348]
Morphine
Chronic morphine administration enhances the expression of Kv1.5 and Kv1.6 voltage-gated K+ channels in rat spinal cord [1762]
Aldosterone
In Wistar rats the application of aldosterone downregulated KCNA5 and other left-ventricular mRNAs coding for Kv4.2, Kv4.3, Kir2.1, Kir2.3 and Cav1.2. Expression of Kv1.5, Kir2.3 and Cav1.2 channels was significantly decreased by aldosterone administration [2018]
Temperature

Anti-FLAG staining revealed the localization of Kv1.5-FLAG in a reticular pattern throughout the cytosol and in a perinuclear pattern throughout the nuclear envelope, indicating its presence in the endoplasmic reticulum (ER) [1589]
Atria Specific
Kv1.5 channels are atria-specific and have thus been proposed as pharmacological targets for antiarrhythmic drugs effective on supraventricular arrhythmias, such as atrial fibrillation. [122]
Human Kv1.5 (hKv1.5) is encoded by KCNA5 and KCNA5 loss-of-function mutations have been reported to produce kindred atrial arrhythmia, which suggest that the genetic alteration of hKv1.5 may substantially enhance arrhythmia susceptibility [1573]
IKur blockers have been shown to affect human atrial AP repolarization in vitro, and selectively increase atrial refractoriness and terminate atrial arrhythmias in several animal species in vivo [426]
Cell Cycle Regulation
Kv1.5 has a crucial function in cell cycle regulation. Kv1.5 subunit is up-regulated during G1 phase of the cell cycle, it is conceivable that this subunit plays a relatively minor role when compared with Kv1.3 in OP G1/S cell cycle progression [1575]
In the heart, Kv1.5 contributes to the repolarization of the cardiac action potential [587] and is responsible for the ultrarapid delayed rectifier current [588].
Kv1.5 also plays an important role in cell membrane potential regulation [1587]
Spermatozoa
Kv1.5, minK and TASK2 were localized to various regions of the spermatozoa. Although Kv1.4, Kv1.7, TASK2 and TASK3 channels may have important roles in human spermatozoa, Kv1.5 and minK appear to be the most likely candidates for human sperm RVD, serving as targets for non-hormonal contraception [1653]
Disease
(PULMONARY HYPERTENSION) Overexpression of Kv1.5 in cultured PASMCs (Pulmonary artery smooth muscle cells) could offset hypoxia-induced cell proliferation and hypoxia-inhibited Kv currents in the IUGR group. These results suggest that the inhibited expression of Kv1.5 in PASMCs contribute to the development of exaggerated CH-PHT (Chronic hypoxia pulmonary hypertension) in rats during adulthood [1586]
(HYPOTHYROIDISM) Kv1.5 is markedly decreased by hypothyroidism and deletion of the Kv1.5-partner kcne2, which impairs channel expression, triggers cardiopathies with a marked form of hypothyroidism with deleterious results in neonates [348]
(CARDIAC ARREST) Low temperature exposure stabilizes the protein in the cellular organelles or on the plasma membrane, and modulates its maturation and trafficking, thus enhancing the currents of hKv1.5 (CARDIAC CHANNEL) and its trafficking defect mutants [1573]
(HYPOXIA PULMONARY VASOCONSTRICTION) Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes [1825]
B-cell proliferation
Kv1.5 channels were identified as determinants of human B-lymphocyte proliferation and migration. The abundance of Kv1.5 channel expression was shown to inversely correlate with the clinical aggressiveness of lymphoma [2017]
Chronic hypoxia pulmonary hypertension
Inhibited expression of Kv1.5 in pulmonary artery smooth muscle cells (PASMCs) contributes to the development of chronic hypoxia pulmonary hypertension. Kv1.5 overexpression in PAMSCs was able to compensate hypoxia-induced cell proliferation in the rat model [1586]
Atrial fibrillation
Kv1.5 channels underlie the voltage-gated atrial-specific potassium current Ikur. Gain-of-function and loss-of-function mutation studies provided evidence that both increased and decreased Kv1.5 channel activity can increase atrial fibrillation (AF) susceptibility. The high prevalence of these deleterious variants of KCNA5 in were reported for AF patients [2019]
Obesity-induced electrophysiological remodeling
Obesity-induced electrophysiological remodeling of the heart was reported to involve protein kinase D-mediated reduction of c-AMP response element-binding protein (CREB). In turn, CREB reduction elicits a decrease of cardiaque Kv1.5 channel expression [2020]
Chronic mechanical stress
Chronic mechanical stress may increase Kv1.5 channel expression in atrial myocytes. The upregulation of Kv1.5 channel expression was suggested to be provoked by the overactivation of integrin signaling upon shear stress [2021]
Adaption of exitable cells
The adaption of excitable cells can occur due to various pathological or physiological challenges. In preoptic GABAergic neurons this electrical remodeling was shown to rely on the upregulation of conduction through Kv1.5 channels.[2024]
Cancer cell survival
Survival of cancer cells under stress depends on the suppression of Kv1.5 subunits. The repression of KCNA5 depends on polycomb group (PcG) proteins BMI-1 and EZH2. Inhibition of these PcGs restores Kv1.5 expression and favors stress-induced death [2025]
Hyperoxia
Cardiotoxic effects of hyperoxia involve the downregulation of Kv1.5 and Kvβ subunit expression. The decrease in cardiac Kv1.5 expression is possibly mediated by SiRT1 [2026]
Hypoxia and 15-LOX
Hypoxia can cause Kv1.5 and Kv2.1 suppression. This effect was suggested to be mediated by increased 15-lipoxygenase (15-LOX) expression. This is supported by the finding that inhibition of 15-lipoxygenase can reverse hypoxia-induced down-regulation of Kv1.5 and Kv2.1 channels in rat cardiac myocytes [2027]
Low temperature stabilization
Low temperature exposure of CHO cells was shown to stabilize the Kv1.5 subunits in organelles or the membrane and to enhance hKv1.5 currents [1573]
Cardiac VSM cells, blood flow and metabolism
A KCNA5 knockout study revealed that Kv1.5 channels, expressed in vascular smooth muscle cells, play a critical role in coupling myocardial blood flow to cardiac metabolism [2036]
Osteosarcoma
Kv1.5 is expressed in osteosarcoma. Silencing of Kv1.5 channels suppressed osteosarcoma progression through multiple signaling pathways [2039]
Ewing sarcoma
The promoter of KCNA5 was found to be methylated in Ewing sarcoma cells. Epigenetic silencing of Kv1.5 expression was found to promote tumor cell proliferation, supporting a role of ion channel dysregulation in tumorigenesis [2040]
Mutation
The related Kv1.5 channel, which does not undergo intrinsic N-type inactivation, also exhibits enhanced slow inactivation when the H463 residue in the S5-P-loop linker structure known as the turret is mutated to a glycine (H463G) [1588]
ALA
α-linolenic acid (ALA), a vegetable marine n-3 polyunsaturated fatty acid, blocked Kv1.5 channels in a time- and voltage-dependent manner. [122]
TEA
Kv1.5 is insensitive to TEA [1575]
Kvβ and Drug competition
Drug binding in the inner cavity of the Kv1.5 channel pore can compete with binding of β-subunits because there is partial overlap in the residues involved in either binding [1574]
Kvβ subunits
Interaction of Kvβ subunits with Kv1.5 controls channel trafficking and integration into the plasma membrane, and modulates activation and inactivation kinetics of the current [1574]
KChIP1/2
co-expression of KChIP2 (or KChIP1) decreases Kv1.5-encoded K(+) currents. Although current densities are reduced, KChIP2 (or KChIP1) co-expression does not affect the time- or voltage-dependent properties of heterologously expressed Kv1.5-encoded K(+) currents [1577]
Kvβ1.2, Kvβ1.3, and Kvβ2.1
These are most widely expressed in the heart. When co-expressed with Kv1.5, they shift the steady-state activation and inactivation curves to more negative potentials [1574]
Kvβ3.1
The heterologous coexpression of human Kv1.5 and Kvβ3.1 subunits in Chinese hamster ovary cells yielded a novel Kv channel mediating very fast inactivating (A-type) outward currents upon depolarization. Thus, the expression of Kvβ3.1 subunits potentially extends the possibilities to express diverse A-type Kv channels in the human brain [1579]
PKA and PKC
In human atria, phosphorylation induced by stimulation of β-adrenoceptors and activation of PKA increases, whereas PKC-mediated phosphorylation decreases IKur amplitude. Stimulation of PKC has little effect on expressed Kv1.5 channels alone; however, co-assembly of Kvβ1.2 with Kv1.5 enhances the response of the channel to PKC activation with a reduction in the K+ current [1574]
Nitric Oxide
Kv1.5 channels are also regulated by NO via a cGMP/PKG-dependent pathway. NO donors such as SNAP (S-nitroso-N-acetylpenicillamine) and L-arginine concentration-dependently decrease IKv1.5 in an expression system, and elevate the plateau in mouse ventricular cells (which unlike human ventricular cardiomyocytes do endogenously express Kv1.5) [1574]
DPO
Diphenyl phosphine oxide (DPO) compounds that are potent frequency-dependent inhibitors of cloned human Kv1.5 (hKv1.5) channels. DPO inhibited hKv1.5 expressed in Chinese hamster ovary cells in a concentration-dependent manner preferentially during channel activation and slowed the deactivating tail current, consistent with a predominant open-channel blocking mechanism. Near complete recovery from washout [1584]
Proteins and Lipids
It has been reported that several regulatory proteins and lipids, such as SAP97, half LIM protein 1, b-accessory protein KChIP2, eicosapentaenoic acid and an antiarrhythmic drug, quinidine, regulate Kv1.5 expression and intracellular trafficking processes [1573]
Bisindolylmaleimide, a blocker of Kv1.5
BIM (V) also did not alter the peak current at low concentrations (0.3, 1 μM) but did at high concentrations (3, 10 μM). High concentrations of BIM, the peak amplitude of current was affected much less than the steady-state current amplitude at the end of the 250-ms depolarizing pulse. The washout of BIM (I) and BIM (V) by perfusion of drug-free solution was obtained within 3 min, and currents were recovered to 84.02 ± 0.04% (n = 6) and 87.11 ± 0.11% (n = 5) of control, respectively [1580]
Vernakalant
Of many recent drugs, Vernakalant (RSD1235) is of special interest because it has recently been approved by the European drug agencies for intravenous conversion of recent onset of AF.101,102. It produces a strong, positive frequency-dependent IKur block with an IC50 of 13 µM at 1 Hz in stably expressed hKv1.5 channels, whereas three-fold higher concentrations are required albeit at 0.25 Hz for open-channel block of expressed hNav1.5 channels [1574]
S9947
A novel Kv1.5 channel blocker, S9947 (2′-(benzyloxycarbonylaminometh- yl)biphenyl-2-carboxylic acid 2-(2-pyridyl)ethyl amide) inhibited the cloned human cardiac potassium channel Kv1.5 expressed in Xenopus oocytes and CHO cells with IC50 values of ~0.6 μM and ~0.4 μM, respectively. Heterologous expression of hKv1.5 in CHO cells and Xenopus oocytes revealed differences in the inactivation of the current (fig 2). This could be due, at least in part, to the higher temperature [1581]
AVE0118
One of the first intensely studied Kv1.5 blockers, AVE0118, shortened APs in preparations from patients in sinus rhythm (SR), and only prolonged the dramatically altered AP in preparations from patients in chronic atrial fibrillation [1585]
Loratadine
Loratadine is a long-acting non-sedating histamine H1-receptor antagonist widely prescribed for treating acute coryza and chronic allergic rhinitis and skin disorders. Loratadine inhibited in a concentration-dependent manner the hKv1.5 current, the apparent affinity being 1.2±0.2 μM. The blockade increased steeply between −30 and 0 mV which corresponded with the voltage range for channel opening, thus suggesting that the drug binds preferentially to the open state of the channel. Excellent washout was also observed [1582]
Local Anaesthetics
The effects of local anaesthetics (Ropivacaine, (R/+) Mepivacaine, and (S/-) Mepivacaine) was tested on Kv1.5. While Ropivacine conveyed concentration dependent block, R/+ and S/- Mepivacine block displayed no apparent time-dependence due to a very fast dissociation rate constant [1583]
Channel trafficking by myosins
Myosin-V motor proteins regulate Kv1.5 channel trafficking in cardiac myocytes. MYO5A and MYO5B mediate functionally distinct and isoform-specific steps in surface trafficking of Kv1.5 channels determining cellular exitibility. [2022]
Antiarrhythmic compound, AVE 0118
The antiarrhythmic compound, AVE 0118, elicits an sympathetic effect in rat small mesenteric arteries. This effect was found to be at least in part mediated by AVE 0118 blocking of neuronal Kv1.5 channels in the medio-adventitial layer [2023]
PKC isoform βII
Protein kinases are involved in the regulation of cardiac ion channels. PKC-dependent regulation of Kv1.5/ Kvβ1.2 channels in the heart is specifically regulated by the PKC isoform βII [2028]
Synapse-associated protein 97 and cortactin
Structural remodeling that includes ion channels is supposed to underlie the abnormal action potentials of Purkinje cells (PC) of the infarcted zone. Synapse-associated protein 97 and actin-binding protein cortactin were found to maintain and normalize Kv1.5 channel conductance of arrythmogenic PCs [2029]
SPAK and OSR1
SPS1-related proline/alanine-rich kinase (SPAK) and oxidative stress-responsive kinase 1 (OSR1) regulate membrane proteins including ion channels. A coexpression study in xenopus oocytes demostrated that both SPAK and OSR1 decrease the surface expression and activity of Kv1.5 channels [2030]
Midazolam
The benzodiazepine Midazolam used in anesthesia may elicit cardiac side effects via Kv1.5 channels. Studies on heterologously expressed Kv1.5 channels revealed Midazolam as an open channel inhibitor of cardiac Kv1.5 channels, exerting an fast onset of block without frequency dependence [2031]
Cortisone and hydrocortisone
The glucocorticoids cortisone and hydrocortisone were shown to rapidly and irreversibly suppress the amplitude of Kv1.5 channel current. However, inhibition properties of cortisone and hydrocortisone differed across the voltage range indicating that they inhibit the Kv1.5 channel differently. Altogether, Kv1.5 channels showed more sensitivity to hydrocortisone than to cortisone [2032]
PKC and AMPK regulation of surface expression
PKC and AMPK activation was shown to reduce Kv1.5 current in heterologous expression assays. These experiments demonstrated that PKC and AMPK kinases both reduce surface expression of Kv1.5 but rely on different molecular mechanisms [2034]
Janus kinase3
Janus kinase 3 was shown to negatively regulate Kv1.5 protein abundance and activity. Being sensitive to ouabain, this reduction was suggested to be mediated by the Na+K+ ATPase [2035]
Propofol
The anesthetic propofol affects a variety of ion channels. Whole-cell patch-clamp recording of CHO cells expressing different hKv1.5 mutants revealed that propofol blocks hKv1.5 by directly interacting with specific amino acids near the selectivity filter or the S6 domain [2037]
Intracellular urate
Urate can be taken up by the cell through the uric acid transporter (UAT). Intracellular urate was shown to enhance Kv1.5 protein expression and activity in cultured mouse atrial myocytes [2038]
Paroxetine
The selective serotonin reuptake inhibitor (SSRI) paroxetine reduces currents of rat Kv1.5 channels expressed in CHO cells. The properties of Kv1.5 current alteration suggest that paroxetine acts as an open-channel blocker [2041]
SGK3
The serum glucocorticoid inducible kinase isoform SGK3 regulates membrane transporters and ion channels. In Xenopus oocytes expressing KCNA5, SGK3 enhanced voltage gated currents. Additional SKG3 expression was found to offset downregulation of Kv1.5 currents by coexpressed Nedd4-2. Together, this indicates SGK3 as a positive regulator of Kv1.5 channel currents [2042]
Sertraline
Sertraline, a selective serotonin reuptake inhibitor (SSRI) may elicit cardiac toxicity via Kv1.5 channels. Whole cell patch clamp recording of CHO cells expressing rat Kv1.5 channels expressed in CHO cells suggest that sertraline acts as an open-channel blocker [2044]
References
Guizy M
et al.
Modulation of the atrial specific Kv1.5 channel by the n-3 polyunsaturated fatty acid, alpha-linolenic acid.
J. Mol. Cell. Cardiol.,
2008
Feb
, 44 (323-35).
Koutsouki E
et al.
Modulation of human Kv1.5 channel kinetics by N-cadherin.
Biochem. Biophys. Res. Commun.,
2007
Nov
9
, 363 (18-23).
Tipparaju SM
et al.
NADPH binding to beta-subunit regulates inactivation of voltage-gated K(+) channels.
Biochem. Biophys. Res. Commun.,
2007
Jul
27
, 359 (269-76).
Williams CP
et al.
Modulation of the human Kv1.5 channel by protein kinase C activation: role of the Kvbeta1.2 subunit.
J. Pharmacol. Exp. Ther.,
2002
Aug
, 302 (545-50).
Philipson LH
et al.
Sequence and functional expression in Xenopus oocytes of a human insulinoma and islet potassium channel.
Proc. Natl. Acad. Sci. U.S.A.,
1991
Jan
1
, 88 (53-7).
Bielanska J
et al.
Voltage-dependent potassium channels Kv1.3 and Kv1.5 in human fetus.
Cell. Physiol. Biochem.,
2010
, 26 (219-26).
Trapani JG
et al.
Effect of external pH on activation of the Kv1.5 potassium channel.
Biophys. J.,
2003
Jan
, 84 (195-204).
Brunner M
et al.
In vivo gene transfer of Kv1.5 normalizes action potential duration and shortens QT interval in mice with long QT phenotype.
Am. J. Physiol. Heart Circ. Physiol.,
2003
Jul
, 285 (H194-203).
Nielsen NH
et al.
Mutations in the Kv1.5 channel gene KCNA5 in cardiac arrest patients.
Biochem. Biophys. Res. Commun.,
2007
Mar
16
, 354 (776-82).
Almquist J
et al.
Modeling the effect of kv1.5 block on the canine action potential.
Biophys. J.,
2010
Nov
3
, 99 (2726-36).
Fedida D
et al.
Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes.
Circ. Res.,
2003
Oct
17
, 93 (744-51).
Gaborit N
et al.
Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart.
J. Physiol. (Lond.),
2007
Jul
15
, 582 (675-93).
Vicente R
et al.
Association of Kv1.5 and Kv1.3 contributes to the major voltage-dependent K+ channel in macrophages.
J. Biol. Chem.,
2006
Dec
8
, 281 (37675-85).
Archer SL
et al.
Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5.
FASEB J.,
2001
Aug
, 15 (1801-3).
McGahon MK
et al.
Kv1.5 is a major component underlying the A-type potassium current in retinal arteriolar smooth muscle.
Am. J. Physiol. Heart Circ. Physiol.,
2007
Feb
, 292 (H1001-8).
Snyders DJ
et al.
A rapidly activating and slowly inactivating potassium channel cloned from human heart. Functional analysis after stable mammalian cell culture expression.
J. Gen. Physiol.,
1993
Apr
, 101 (513-43).
Feng J
et al.
Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes.
Circ. Res.,
1997
Apr
, 80 (572-9).
Martens JR
et al.
Modulation of Kv channel alpha/beta subunit interactions.
Trends Cardiovasc. Med.,
1999
Nov
, 9 (253-8).
Ding WG
et al.
Improved functional expression of human cardiac kv1.5 channels and trafficking-defective mutants by low temperature treatment.
PLoS ONE,
2014
, 9 (e92923).
Ravens U
et al.
Ultra-rapid delayed rectifier channels - molecular basis and therapeutic implications.
,
2010
Dec
15
, ().
Chittajallu R
et al.
Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle.
Proc. Natl. Acad. Sci. U.S.A.,
2002
Feb
19
, 99 (2350-5).
Andér M
et al.
Ligand binding to the voltage-gated Kv1.5 potassium channel in the open state--docking and computer simulations of a homology model.
Biophys. J.,
2008
Feb
1
, 94 (820-31).
Li H
et al.
KChIP2 modulates the cell surface expression of Kv 1.5-encoded K(+) channels.
J. Mol. Cell. Cardiol.,
2005
Jul
, 39 (121-32).
Honore E
et al.
External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids.
Proc. Natl. Acad. Sci. U.S.A.,
1994
Mar
1
, 91 (1937-41).
Leicher T
et al.
Coexpression of the KCNA3B gene product with Kv1.5 leads to a novel A-type potassium channel.
J. Biol. Chem.,
1998
Dec
25
, 273 (35095-101).
Choi BH
et al.
Direct block by bisindolylmaleimide of rat Kv1.5 expressed in Chinese hamster ovary cells.
J. Pharmacol. Exp. Ther.,
2000
May
, 293 (634-40).
Bachmann A
et al.
Characterization of a novel Kv1.5 channel blocker in Xenopus oocytes, CHO cells, human and rat cardiomyocytes.
Naunyn Schmiedebergs Arch. Pharmacol.,
2001
Nov
, 364 (472-8).
Delpón E
et al.
Block of human cardiac Kv1.5 channels by loratadine: voltage-, time- and use-dependent block at concentrations above therapeutic levels.
Cardiovasc. Res.,
1997
Aug
, 35 (341-50).
Longobardo M
et al.
Structural determinants of potency and stereoselective block of hKv1.5 channels induced by local anesthetics.
Mol. Pharmacol.,
1998
Jul
, 54 (162-9).
Lagrutta A
et al.
Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current.
J. Pharmacol. Exp. Ther.,
2006
Jun
, 317 (1054-63).
Wettwer E
et al.
Pharmacology of voltage-gated potassium channel Kv1.5-impact on cardiac excitability.
Curr Opin Pharmacol,
2014
Mar
12
, 15C (115-121).
Lv Y
et al.
Decreased Kv1.5 expression in intrauterine growth retardation rats with exaggerated pulmonary hypertension.
Am. J. Physiol. Lung Cell Mol. Physiol.,
2013
Dec
, 305 (L856-65).
Mia S
et al.
Downregulation of Kv1.5 K Channels by the AMP-Activated Protein Kinase.
Cell. Physiol. Biochem.,
2012
Sep
24
, 30 (1039-1050).
Cheng YM
et al.
ShakerIR and Kv1.5 mutant channels with enhanced slow inactivation also exhibit K⁺ o-dependent resting inactivation.
Pflugers Arch.,
2013
Nov
, 465 (1545-55).
Hirota Y
et al.
Functional stabilization of Kv1.5 protein by Hsp70 in mammalian cell lines.
Biochem. Biophys. Res. Commun.,
2008
Aug
1
, 372 (469-74).
Barfield JP
et al.
Characterization of potassium channels involved in volume regulation of human spermatozoa.
Mol. Hum. Reprod.,
2005
Dec
, 11 (891-7).
Matus-Leibovitch N
et al.
Chronic morphine administration enhances the expression of Kv1.5 and Kv1.6 voltage-gated K+ channels in rat spinal cord.
Brain Res. Mol. Brain Res.,
1996
Sep
1
, 40 (261-70).
Gebauer M
et al.
N-type inactivation features of Kv4.2 channel gating.
Biophys. J.,
2004
Jan
, 86 (210-23).
Archer SL
et al.
Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes.
J. Clin. Invest.,
1998
Jun
1
, 101 (2319-30).
Vallejo-Gracia A
et al.
Emerging role for the voltage-dependent K+ channel Kv1.5 in B-lymphocyte physiology: expression associated with human lymphoma malignancy.
J. Leukoc. Biol.,
2013
Oct
, 94 (779-89).
Dartsch T
et al.
Aldosterone induces electrical remodeling independent of hypertension.
Int. J. Cardiol.,
2013
Apr
5
, 164 (170-8).
Christophersen IE
et al.
Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation.
Eur. Heart J.,
2013
May
, 34 (1517-25).
Huang H
et al.
Diet-induced obesity causes long QT and reduces transcription of voltage-gated potassium channels.
J. Mol. Cell. Cardiol.,
2013
Jun
, 59 (151-8).
Boycott HE
et al.
Shear stress triggers insertion of voltage-gated potassium channels from intracellular compartments in atrial myocytes.
Proc. Natl. Acad. Sci. U.S.A.,
2013
Oct
8
, 110 (E3955-E3964).
Schumacher-Bass SM
et al.
Role for myosin-V motor proteins in the selective delivery of Kv channel isoforms to the membrane surface of cardiac myocytes.
Circ. Res.,
2014
Mar
14
, 114 (982-92).
Kun A
et al.
Neurogenic Contraction Induced by the Antiarrhythmic Compound, AVE 0118, in Rat Small Mesenteric Arteries.
Basic Clin. Pharmacol. Toxicol.,
2014
Mar
14
, ().
Tabarean IV
Electrical remodeling of preoptic GABAergic neurons involves the Kv1.5 subunit.
PLoS ONE,
2014
, 9 (e96643).
Ryland KE
et al.
Polycomb-dependent repression of the potassium channel-encoding gene KCNA5 promotes cancer cell survival under conditions of stress.
Oncogene,
2015
Aug
27
, 34 (4591-600).
Chapalamadugu KC
et al.
High level of oxygen treatment causes cardiotoxicity with arrhythmias and redox modulation.
Toxicol. Appl. Pharmacol.,
2015
Jan
1
, 282 (100-7).
Liu W
et al.
Inhibition of 15-lipoxygenase (15-LOX) reverses hypoxia-induced down-regulation of potassium channels Kv1.5 and Kv2.1Inhibition of 15-lipoxygenase (15-LOX) reverses hypoxia-induced down-regulation of potassium channels Kv1.5 and Kv2.1.
Int J Clin Exp Med,
2014
, 7 (4147-53).
Fischer F
et al.
Isoenzyme-specific regulation of cardiac Kv1.5/Kvβ1.2 ion channel complex by protein kinase C: central role of PKCβII.
Naunyn Schmiedebergs Arch. Pharmacol.,
2014
May
, 387 (469-76).
Dun W
et al.
SAP97 and cortactin remodeling in arrhythmogenic Purkinje cells.
PLoS ONE,
2014
, 9 (e106830).
Elvira B
et al.
SPAK and OSR1 sensitivity of voltage-gated K+ channel Kv1.5.
J. Membr. Biol.,
2015
Feb
, 248 (59-66).
Vonderlin N
et al.
Inhibition of cardiac Kv1.5 potassium current by the anesthetic midazolam: mode of action.
Drug Des Devel Ther,
2014
, 8 (2263-71).
Yu J
et al.
Inhibitory effects of cortisone and hydrocortisone on human Kv1.5 channel currents.
Eur. J. Pharmacol.,
2015
Jan
5
, 746 (158-66).
Andersen MN
et al.
PKC and AMPK regulation of Kv1.5 potassium channels.
Channels (Austin),
2015
, 9 (121-8).
Warsi J
et al.
Regulation of Voltage-Gated K(+) Channel Kv1.5 by the Janus Kinase JAK3.
J. Membr. Biol.,
2015
Jun
23
, ().
Ohanyan V
et al.
Requisite Role of Kv1.5 Channels in Coronary Metabolic Dilation.
Circ. Res.,
2015
Sep
11
, 117 (612-21).
Kojima A
et al.
Interaction of propofol with voltage-gated human Kv1.5 channel through specific amino acids within the pore region.
Eur. J. Pharmacol.,
2015
Oct
5
, 764 (622-32).
Maharani N
et al.
Molecular Mechanisms Underlying Urate-Induced Enhancement of Kv1.5 Channel Expression in HL-1 Atrial Myocytes.
Circ. J.,
2015
, 79 (2659-68).
Wu J
et al.
Silencing of Kv1.5 Gene Inhibits Proliferation and Induces Apoptosis of Osteosarcoma Cells.
Int J Mol Sci,
2015
, 16 (26914-26).
Ryland KE
et al.
Promoter Methylation Analysis Reveals That KCNA5 Ion Channel Silencing Supports Ewing Sarcoma Cell Proliferation.
Mol. Cancer Res.,
2016
Jan
, 14 (26-34).
Lee HM
et al.
Blockade of Kv1.5 by paroxetine, an antidepressant drug.
Korean J. Physiol. Pharmacol.,
2016
Jan
, 20 (75-82).
Ahmed M
et al.
SGK3 Sensitivity of Voltage Gated K+ Channel Kv1.5 (KCNA5).
Cell. Physiol. Biochem.,
2016
, 38 (359-67).
Orts DJ
et al.
Biochemical and electrophysiological characterization of two sea anemone type 1 potassium toxins from a geographically distant population of Bunodosoma caissarum.
Mar Drugs,
2013
Mar
, 11 (655-79).
Lee HM
et al.
Blockade of Kv1.5 channels by the antidepressant drug sertraline.
Korean J. Physiol. Pharmacol.,
2016
Mar
, 20 (193-200).
Contributors: Rajnish Ranjan, Michael Schartner, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/5/ , accessed on 2025 Nov 11