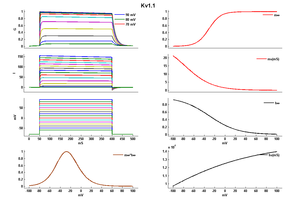
Kv1.1
Description: potassium voltage-gated channel, shaker-related subfamily, member 1 Gene: Kcna1 Alias: Kv1.1, kcna1, kcpvd
Kv1.1, encoded by the gene KCNA1, is a member of the potassium voltage-gated channel subfamily A. In the nervous system Kv1.1 appears to be widely distributed but it is also expressed in unmyelinated axons, cell somas, axon terminals, and in some dendrites [734]. Disruption of the KCNA1 gene in mice leads to frequent spontaneous seizures [1345].
Experimental data
Rat Kv1.1 gene in CHO host cells datasheet |
||
|
Click for details 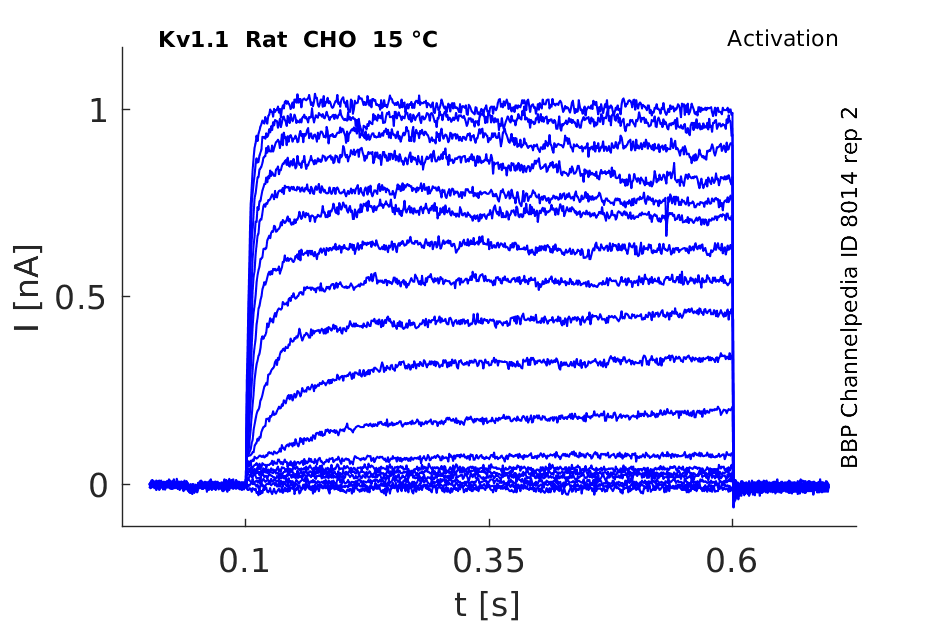
15 °Cshow 106 cells |
Click for details 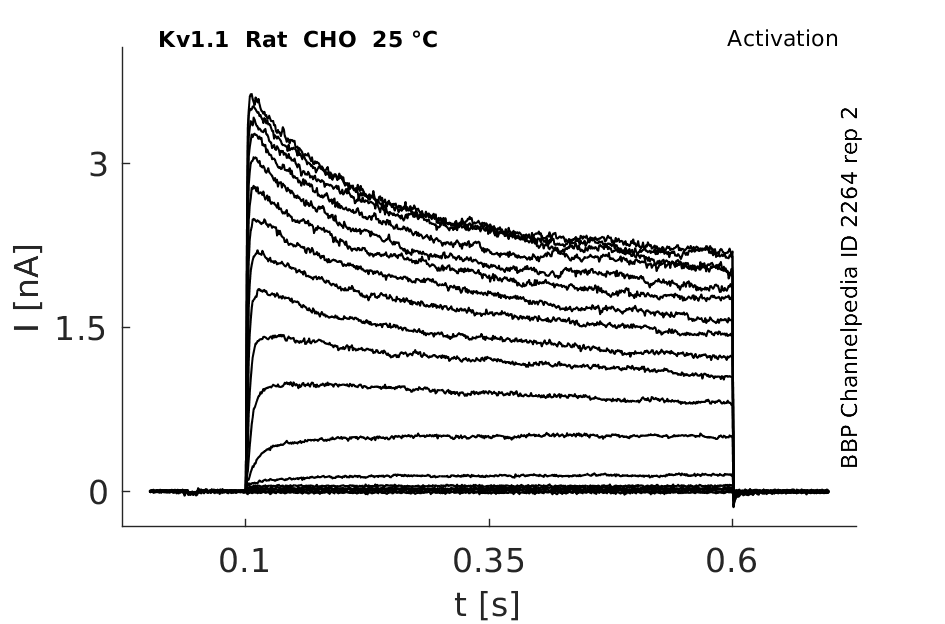
25 °Cshow 237 cells |
Click for details 
35 °Cshow 113 cells |
Mouse Kv1.1 gene in CHO host cells datasheet |
||
|
Click for details 
25 °Cshow 86 cells |
Click for details 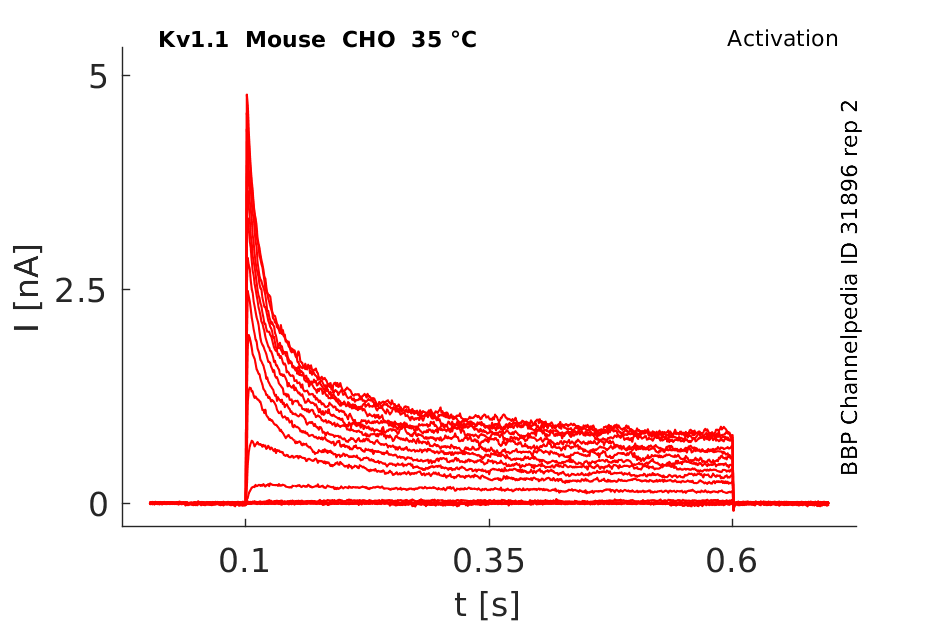
35 °Cshow 44 cells |
|
Human Kv1.1 gene in CHO host cells datasheet |
||
|
Click for details 
25 °Cshow 127 cells |
Click for details 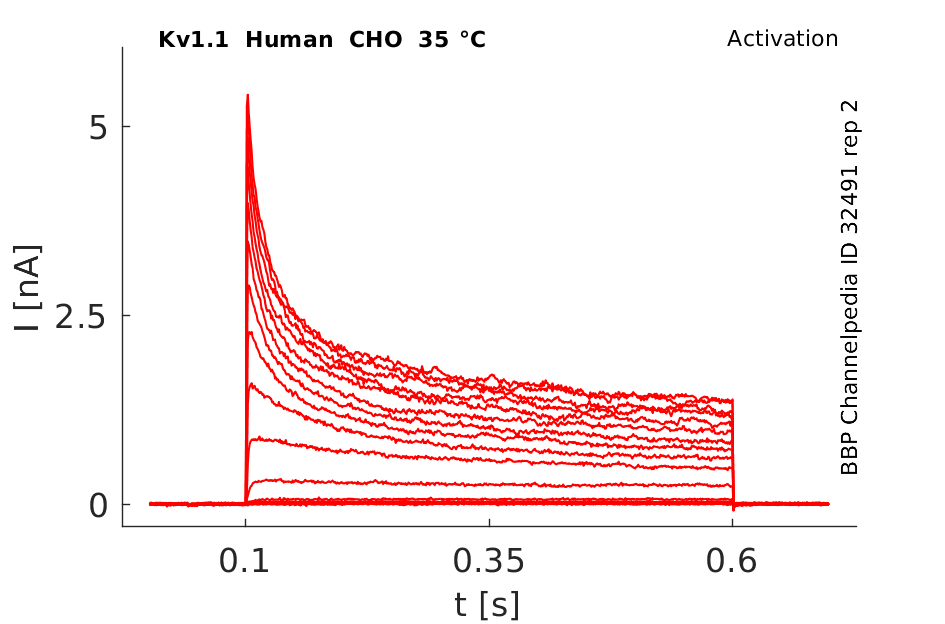
35 °Cshow 58 cells |
|
Rat Kv1.1 gene in HEK host cells datasheet |
||
|
Click for details 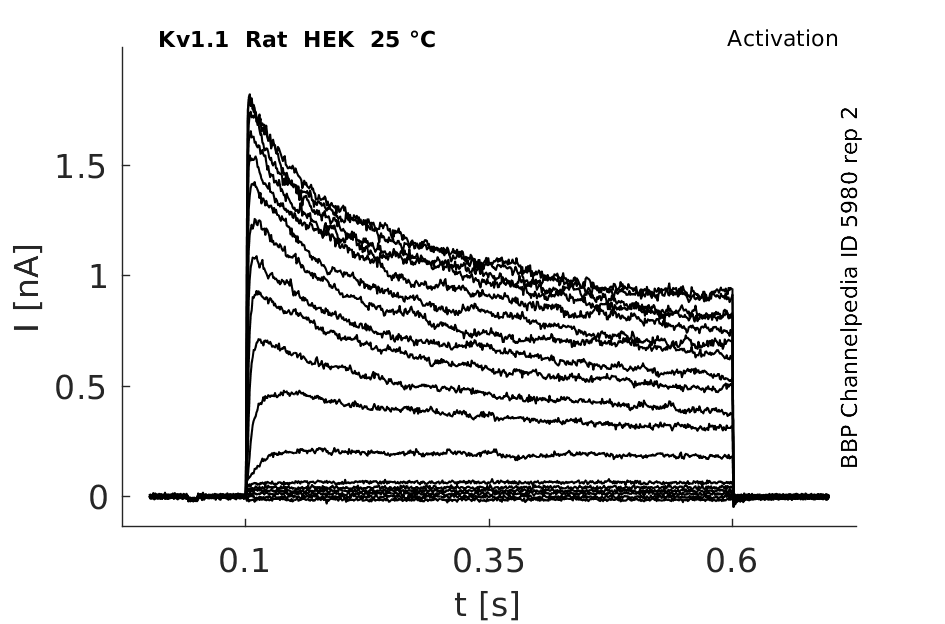
25 °Cshow 112 cells |
Click for details 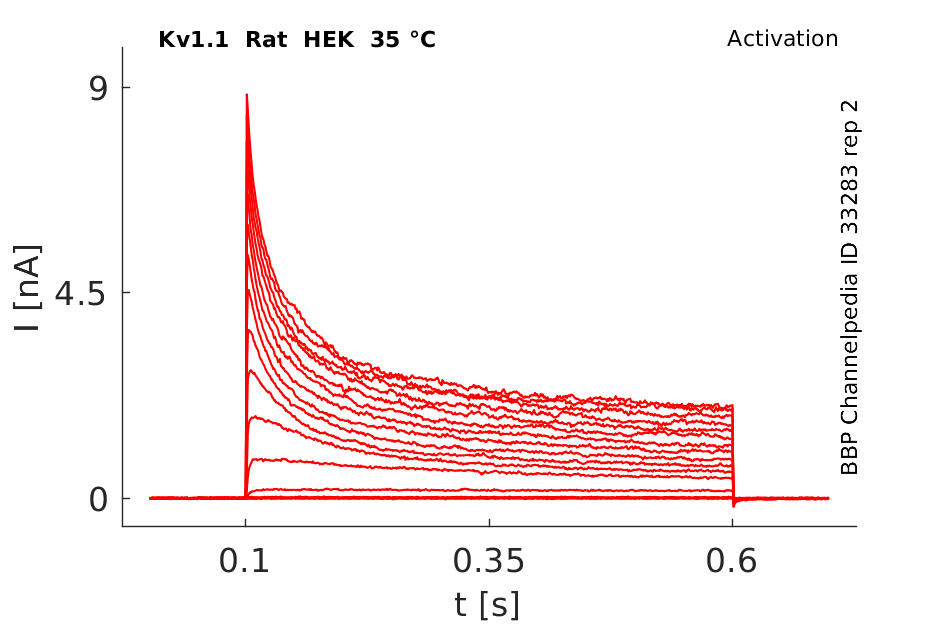
35 °Cshow 59 cells |
|
Rat Kv1.1 gene in CV1 host cells datasheet |
||
|
Click for details 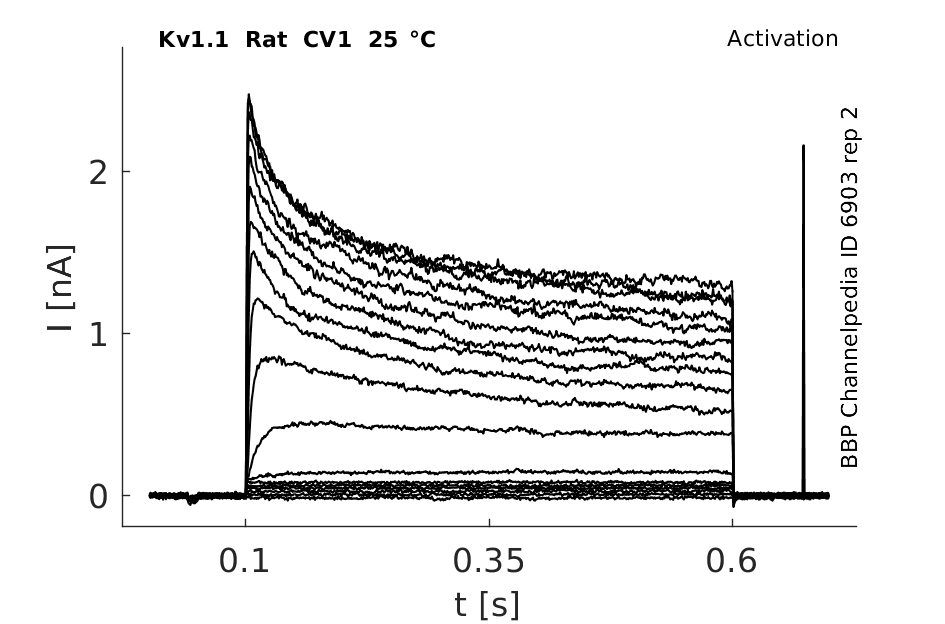
25 °Cshow 124 cells |
Click for details 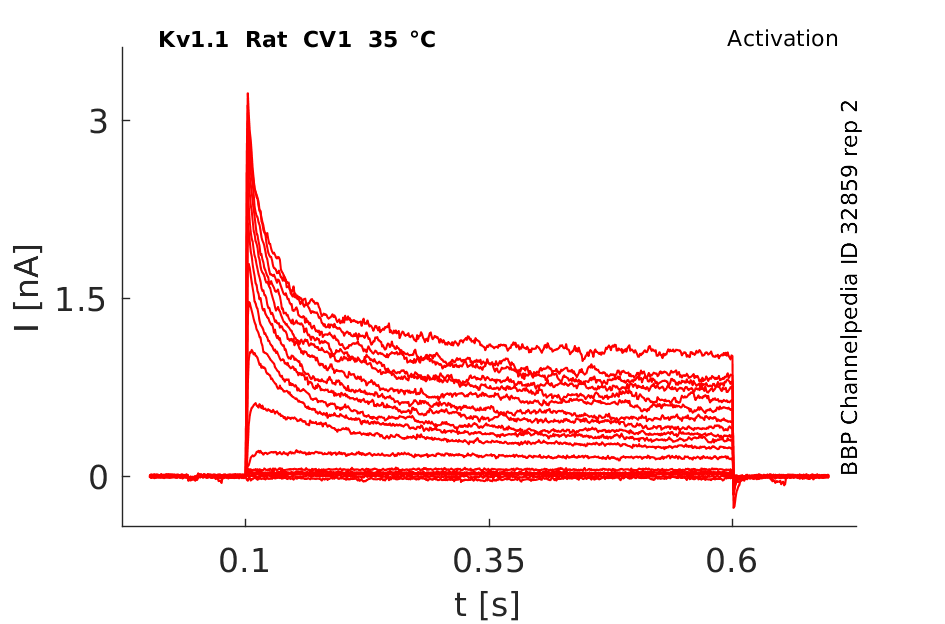
35 °Cshow 109 cells |
|
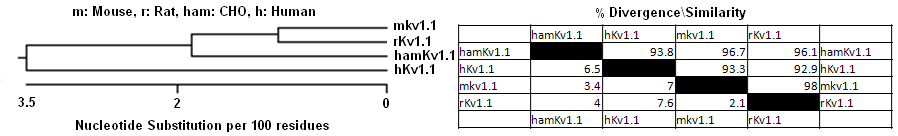
The Kcna1 gene is conserved in human, chimpanzee, dog, cow, mouse, chicken and zebrafish. It contains only one exon hence no splicing event possible.
Phylogeny
Variants
No splicing variants of kcna1 are known.
Kcna1 mRNA is subject to RNA editing[371].
| Species | NCBI accession | Length (nt) | |
|---|---|---|---|
| Human | NM_000217.3 | 7985 | |
| Mouse | NM_010595.3 | 8970 | |
| Rat | NM_173095.3 | 1746 |
Isoforms
Post-Translational Modifications
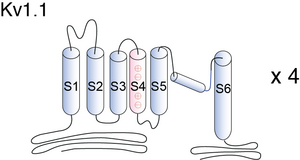
Visual Representation of Kv1.1 Structure
Methodology for visual representation of structure available here
Crystal Structure
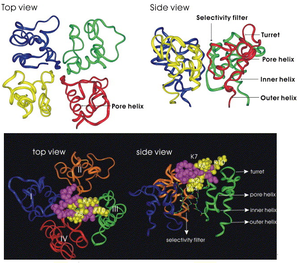
Pharmacological Characteristics
Pharmacological characteristics of Kv1.1- and Kv1.2-containing channels are influenced by the stoichiometry and positioning of their α subunits.The findings of the present study support the possibility of α subunits being precisely arranged in Kv1 channels, rather than being randomly assembled. This is important in designing drugs with abilities to inhibit particular oligomeric Kv1 subtypes, with the goal of elevating neuronal excitability and improving neurotransmission in certain diseases [1603]
Kv1.1 predicted AlphaFold size
Methodology for AlphaFold size prediction and disclaimer are available here
SIngle channel current in CHO
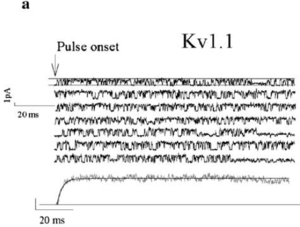
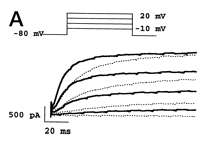
Rat Kv1.1 in CHO Cells
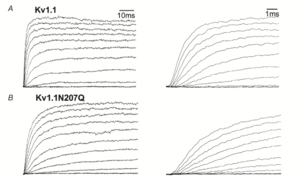
Kv1.1 kinetics
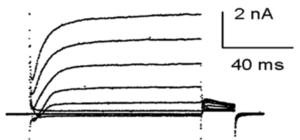
Kinetics of mKv1.1 in X. oocytes
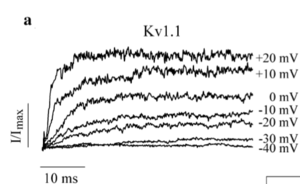
Kv1.1/Kv1.4 channels and mutants recorded in Xenopus oocytes
In Kv1.1/Kv1.4 channels, the Kv1.4 subunits refer rapid inactivation properties to otherwise noninactivating Kv1.1 channels via their N-terminal inactivation domains. Normalized whole cell currents were recorded at +60 mV from X. laevis oocytes coexpressing Kv1.4WT and Kv1.1EA1 mutants and compared to WT control traces. The results showed that the N-type inactivation rates of Kv1.1/Kv1.4 channels is faster in I177N and E325D mutations and slower in channels with the V404I and V408A mutations.[1886]
hKv1.1 currents in CHO cells and inhibition by urotoxin
Whole-cell hKv1.1 currents were measured in Chinese hamster ovary (CHO) cells during a voltage pulse to +60 mV for 300 ms, and a repolarizing step to 250 mV for 100 milliseconds at intervals of 2.7 seconds. hKv1.1 currents were fast and reversibly inhibited in the presence of 1 mM urotoxin.[1889]
Kv1.1 currents in HEK cells and effect of EA1-associated mutation
HEK293 cells were transiently transfected with Kv1.1 channels and mutant variants. Currents were elicited by application of square voltage steps from -80 mV to +50 mV in 200 ms intervals from a holding voltage of -80 mV. Tail currents were evoked by extracellularly added potassium. In WT Kv1.1-expressing cells fast delayed rectifying currents were recorded at depolarizing potentials and density currents of 186 +- 42 pA/pF were measured. In Kv1.1 I262M-mutant expressing cells currents were significantly (P < 0.05) reduced with recorded density currents of 14 +- 3 pA/pF. The mutated variant elicits a dominant negative effect when co-expressed with WT Kv1.1.[1908]
Comparison of Kv1.1 Wt and Kv1.1 F184C currents in Xenopus oocytes
The Mutation F184C in Kv1.1 associated with of episodic ataxia type I (EA1) impaires fast and slow inactivation of Kv1.1.[1884]
rKv1.1 currents in Xenopus oocytes and effects of scorpion venom K blockers
Rat Kv1.1 currents were measured in Xenopus oocytes. Further, the effects on the rKv1.1 by potassium channel-blocking toxins from the Mesobuthus eupeus scorpion venom were measured in Xenopus oocytes. Using the two-electrode voltage clamp technique eight high affinity Kv1.1 channel blockers were identified.[2062]
Markov Model
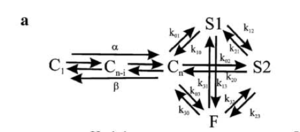
Hodgkin and Huxley type model
Model Kv1.1 (ID=18)
Model was built from [252]
| Animal | rat | |
| CellType | Oocyte | |
| Age | 0 Days | |
| Temperature | 24.0°C | |
| Reversal | -65.0 mV | |
| Ion | K + | |
| Ligand ion | ||
| Reference | [271] J P Adelman et. al; Science 1989 Apr 14 | |
| mpower | 1.0 | |
| m Inf | 1.0000/(1+ exp((v - -30.5000)/-11.3943)) | |
| m Tau | 30.0000/(1+ exp((v - -76.5600)/26.1479)) | |
| hpower | 2.0 | |
| h Inf | 1.0000/(1+ exp((v - -30.0000)/27.3943)) | |
| h Tau | 15000.0000/(1+ exp((v - -160.5600)/-100.0000)) | |
Mammalian Brain
Kv1.1 is widely expressed in mammalian brain but rare in mouse myocardium.[346]
CNS
Although broadly expressed throughout many regions of the central nervous system—including hippocampus, cerebellum, and brainstem nuclei—differential expression of Kv channels facilitates the organization and transfer of neuronal information [1590]
BRAIN
Hippocampal expression of Kv1.1, Kv1.2, and Kv1.4 channels occurs in the middle molecular layer of the den- tate gyrus, where the channels are highly expressed in the axons and terminals of the medial perforant path. Localization to subpopulations of interneurons was found in hilus and CA1 [1590]
Kidney
Renal expression of Kv1.1 was attested for cells of the distal convoluted tubule and associated with Mg2+ re-absorbation [1885]
Heart
Kv1.1 expression in human atria suggests a cardiac role. Kv1.1 deficiency may elicit a higher susceptibility to atrial fibrillation (AF)[1920]
CO-LOCALIZATION INFLUENCES NEURONAL DISTRIBUTION
Assembly of Kv1.1 with Kv1.4 prevents axonal localization but not surface expression, while inclusion of Kv1.2 imparts clustering at presynaptic sites and decreases channel mobility within the axon [1350].
DISTRIBUTION OF KV1.1 in NEURONS
When exogenously expressed in neurons, Kv1 proteins are detected in both dendrites and axons. However, detection of surface-expressed channels has revealed that Kv1 channels are only expressed in the axonal membrane. This has led to the speculation that Kv1 may be localized to the axonal surface through compartment-specific endocytosis or compartment-specific vesicle fusion. Conversely, recent studies have indicated that axonal targeting of Kv1 channels is mediated by selective transport to the axon. [1351]
AIS
Highly dense clusters of Kv1.1 and Kv1.2 are found in the juxtaparanodal (JPX) region next to the nodes of Ranvier (NORs), at axonal initial segments (AIS), and in the pinceau region terminals of cerebellar basket cells. Hippocampal expression of Kv1.1, Kv1.2, and Kv1.4 channels occurs in the middle molecular layer of the dentate gyrus, where the channels are highly expressed in the axons and terminals of the medial perforate path [1590]
Kv1.1 and Kv1.2 subunits show identical distribution along the AIS of neurons from the forebrain and cerebellum of adult male Wistar rats when both are present together, which may mean that they share the same anchoring machinery and may form heteromeric channels. A couple of anchoring proteins including Caspr2 [441] and PSD-93 [427] were implicated in clustering Kv1 channels in the AIS. Recent experiments show that homomeric Kv1.1 channels are retained in the endoplasmic reticulum [453], which support the hypothesis of heteromeric assembly [329].
Expression of Kv1.1
Both endogenous Kv1.1 mRNA and newly synthesized Kv1.1 protein were found prominently in dendrites associated with translational “hotspots.” A unique aspect of local mRNA translation in dendrites is the selective localization of protein synthesis machinery at or near synaptic sites and an accumulation of mRNAs near synaptic sites that have recently experienced strong activity, a form of synaptic tagging. That Kv1.1 mRNA was found at such sites prompted Rabb-Graham et al. to test whether translation of this component subunit of the Kv1 channels, critical to regulating dendritic excitability, could be regulated by neuronal activity, as has been shown for a subset of other dendritic proteins [1770]
RNA editing and trafficking
RNA editing of a single amino acid (I400V)located in the inner pore cavity of Kv1.1 was shown to reduce surface expression of Kv1.1 and whole-cell currents. Kv1.4 expression was not subject to this trafficking regulation [1898]
Neuronal Control
A fast delayed rectifying K+ channel in heterologous expression systems is encoded by Kv1.1. Neuronal deficiency or blockade of Kv1.1 influenced learning negatively or increased transmitter release, respectively, which implies the role of Kv1.1 in repolarizing the membrane in axons and at synaptic terminals and impulse conduction. [329] [1592]
Kv1.1-containing channels are critical for temporal precision during spike initiation [1772]
Mice lacking Kcna1-gene-encoded Kv1.1 shaker-like potassium channels exhibit severe seizures and die prematurely. [346] Recordings from neurons in Kcna1-null animals demonstrated that less current was required to produce AP firing and that these Kv1.1-null neurons typically fired many APs compared with the single AP observed in wildtype mice [1349]. These results indicate that Kv1.1 plays a critical role in setting both the point at which a neuron generates an AP and for a given stimulus whether the cell fires single or multiple APs.
Kv1 channels have a defined task in shaping the AP of presynaptic compartments in the sub millisecond range [1595]
In DRG neurons the inhibition of Kv1.1 produces a nearly twofold increase in the number of APs evoked by a ramp of depolarizing current. Associated with the increased AP firing were reductions in both the firing threshold and the rheobase[252].
Deminished expression of the Kv1.1 channel subunit in parvalbumin-positive, fast-spiking interneurons (FSI) coincides with their reduced first-spike latency. This dysfunction, reported after transient disruption of NMDA signaling during neonatal development, likely contributes to the pathophysiology of Schizophrenia [1900]
Axonal Kv1.1 channels in CA3 neurons determine glutamate release in a time-dependent manner through the control of the presynaptic spike waveform [1911]
Small pyramidal neurons in layer 2 of the rodent granular retrosplenial cortex (GRS), associated with head-direction cells, exhibit a late-spiking (LS) firing property as a consequence of delayed rectifier and A-type potassium channels (Kv1.1, Kv1.4 and Kv4.3)[1891]
Kv1.1 channels shape the firing characteristics of single-spiking Mauthner cells, which are reticulospinal neurons (RSNs) in the hindbrain of embryonal zebrafish involved in escape behaviour[1892] The aquisition of these unique firing properties requires the coexpression of Kvβ2 subunits along with Kv1.1 channels [1902]
AIS
Experiments found that in cells where the Kv1.1 and Kv1.2 subunits are coexpressed with the Nav1.6 subunit, their subcellular distributions are correlated. For instance, in AISs where the Nav1.6 subunit distribution shows a strong gradient, the Kv1.1/Kv1.2 subunits also increase in density toward the distal part of the AIS [329].
The presence of Kv1 channels at the AIS has been demonstrated in a variety of cell types, where they have been shown to play important roles in regulating the timing of AP initiation and AP firing patterns [1590]
Input deprivation switched Kv channel expression at the AIS from Kv1.1 to Kv7.2 enhancing neuronal excitability [1910]
The transmembrane disintegrin ADAM11 is essential for the localization of Kv1.1 and Kv1.2 subunit complexes to the specialized axonal ending of the basket cell (pinceau) encapsulating the Purkinje AIS. Absence of Kv1 channels at the Baskets cells distal terminal due to Adam11 mutation eliminates the ultrarapid ephaptic inhibitory synchronization of Purkinje cell firing [1915]
Seizure, Epilepsy and Ataxia
Kcna1 and Kcna2, is associated with neu- rologic pathologies including epilepsy and ataxia in humans and in rodent models. Kv1.1 and Kv1.2 knockout mice both have seizures beginning early in development; however, each express a different seizure type (pathway) [1590] Characterization of the Kv1.1 I262T and S342I mutations associated with episodic ataxia 1 with distinct phenotypes [1767]Episodic ataxia associated mutations alter subunit surface expression of Kv1 heteromers [1886]
Seizure onset in KCNA1 null mice was affected by circadian rythmicity as demonstrated by epidural EEG [1917]
In a cohort study of 39 participants with clinical phenotype of AE1 10 different pathogenic point mutations in KCNA1 were identified accounting for 85% of the subjects [1907] A point mutation of Kv1.1(I262M) causing phenotypic EA1 was shown to lead to a defective channel in HEK cell expression system [1908]
The combination of copy number variants (CNVs) of KCNA1 and single nucleotide polymorphisms (SNPs) of SCN1A may constitute a principal risk factor for sudden unexpected death in epilepsy (SUDEP)[1906] Physiological examination of KCNA -/- mice suggests a role for the vagus nerve in mediating seizure related bradycardy and SUDEP [1909] Spreading depolarization in the brainstem of KCNA -/- mouse may cause sudden cardiorespiratory arrest[1913]
RNA editing of the Kv1.1 mRNA at the functionally relevant I/V site was found to be inversely correlated to the duration of epileptic seizures in patients [1895]
Spontaneous seizures in Kcna1-null mice activate select limbic circuits, as revealed by C-Fos immunohistochemistry, suggesting the recruitment of extrahippocampal networks in particular the amygdala and hilus during epilepsy[1921]
Hearing impairment
A study of the genetic basis of episodic ataxia type 1 in 15 individuals of four families_reports new mutations that all caused a loss of K(v)1.1 channel function. 4 cases of deafness in this group suggest a possible link between Kv1.1 dysfunction and hearing impairment [1897]
Behavioral effect of Kv1.1 deletion impedes primarily binaural integration and mimics monaural hearing [1919]
Pain Sensation
Finally, mice lacking Kv1.1 channels exhibit signs of neuronal hyperexcitability such as spontaneous seizures and enhanced pain sensation [1592] Kv1.1 channels act as mechanical brake in the senses of touch and pain [1765]
Senescense
Potassium channel KCNA1 modulates oncogene-induced senescence and transformation [1764]
Hypomagnesemia
Functional analysis of the Kv1.1 N255D mutation associated with autosomal dominant hypomagnesemia [1769] N255D mutation of KCNA1 results in a nonfunctional channel compromising Mg2+ re-absorption in cells of the kidneys distal convoluted tubule [1885]
Hyperthermia, Migraine
A single nucleotide change in KCNA1 (c.555C>G) changes a highly conserved residue (p.C185W) of Kv1.1 and may cause a dominant-negative phenotype including hyperthermia and severe migraine [1918]
Neuronal Activity and Cell Volume
Kv1.1 blockade may target neurons and astrocytes, and modulate neuronal activity and neural cell volume, which may partly account for the attenuation of the neurological deficits. We propose that Kv1.1 blockade has a broad therapeutic potential in neuroinflammatory diseases (multiple sclerosis, stroke, and trauma).
Ketogenic diet (KD)attenuates CA3-generated pathologic oscillations presumably by dampening hyperactive mossy fiber in Kv1.1 alpha KO mice [1888]
Knock Out
Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices [1766]
Kcna1 gene deletion lowers the behavioral sensitivity of mice to small changes in sound location and increases asynchronous brainstem auditory evoked potentials but does not affect hearing threshold [1768]
KCNA1 null mice also displayed vagus nerve related bradycardie [1909]
Mutation
Mutation of position F184 of the Shaker channel shows that F184 interacts with the gating charges of S4 subunit and creates a functional link to the selectivity filter of the neighboring subunit.[1884]
Tetraethylammonium (TEA)inhibits Kv1.1 by binding Tyr379 in the pore region. Mutagenesis of Kv1.2 to resemble Kv1.1 and the ratio of subunit assembly of Kv1.1 and Kv1.2 were shown to alter TAE susceptiblity [1603]
In rats carrying a missence mutation in the S4 voltage-sensor domain of KCNA1 myokymia,neuromyotonia and generalized tonic–clonic seizures are the dominant phenotypes displayed [1887]
A C-terminus-truncated mutant of Kv1.1 is associated with severe drug-resistant episodic ataxia type 1. Cultured rat hippocampal neurons expressing this mutant displayed increased excitability and neurotransmitter release probability compared to those trancduced with the wild type gene coding for Kv1.1 [1903]
Transcriptional Regulation
Evidence for combinatorial regulation of gene expression of the Drosophila Shaker channel homolog of Kv1.1 by Isl and Lim3 has come from experiments using DNA adenine methyltransferase identification (DamID)[1901]
miRNA repression
Kv1.1 mRNA translation is repressed by miR-129 in the presence of mTORC1 kinase activity. RNA-binding protein HuD promotes Kv1.1 mRNA translation in the absence of mTORC1 activity [1894] Post status epilepticus Kv1.1 was proposed to be repressed in two phases; an initial mTOR-dependent repression sensitive to rapamycin and after 21 days through persistent inhibition by miR-129-5p [1905]
Ca2+/Calmodulin
Calcium-dependend binding of calmodulin to Kvβ1.1 decelerates the Kvβ1.1 induced inactivation of Kv1.1 and Kv1.4 channels [1916]
Amitriptyline and Retigabine
Amitriptyline inhibited Kv1.1 and Kv7.2/7.3 channels in a concentration-dependent and reversible manner. The IC50-value was 22 +/- 3 microM (n = 33) and 10 +/- 1 microM (n = 40), respectively. Since amitriptyline inhibited Kv1.1 and Kv7.2/7.3 channels only at toxicologically relevant plasma concentrations, our results suggest a role for these channels in the neuroexcitatory side effects of amitriptyline. As the inhibitory effects of amitriptyline were reversed by retigabine, a combination of amitriptyline and retigabine could be of additional benefit in the therapy of neuropathic pain [1707]
Kv Family
Studies have demonstrated that in the nervous system Kv1.1 can combine with KV1.2, 1.4 or Kv1.6 [432]. However homomers of Kv1.1 have not been detected in the nervous system[432].
Syntaxin 1A
Single Kv1.1 channels interact with syntaxin 1A in the following way: Syntaxin decreases the unitary conductance of all conductance states (two subconductances and a full conductance) and decreases their open probabilities by prolongation of mean closed dwell-times at depolarized potentials. Syntaxin 1A increases the probabilities of the subconductance states at subthreshold potentials. This leads to decrease of the macroscopic conductance at potentials above threshold and increase of it at threshold potentials. [1]
Fatty acid ethyl esters
fatty acid ethyl esters, dramatically accelerate the kinetics of the voltage-induced activation of the human brain delayed rectifier potassium channel, Kv1.1. Specifically, the external application of ethyl oleate (20 μM) to Sf9 cells expressing the recombinant Kv1.1 channel resulted in a decrease in the rise times of the macroscopic current [1591]
Kvbeta1 and Kvbeta2 beta-subunits with Kv1
A much larger portion of the total brain pool of Kv1-containing channel complexes was found associated with Kvbeta2 than with Kvbeta1. Single- and multiple-label immunohistochemical staining indicated that Kvbeta1 codistributes extensively with Kv1.1 and Kv1.4 in cortical interneurons, in the hippocampal perforant path and mossy fiber pathways, and in the globus pallidus and substantia nigra. This suggests Kvbeta1 and Kvbeta2 associate and colocalize with Kv1 alpha-subunits in native tissues and provide a biochemical and neuroanatomical basis for the differential contribution of Kv1 alpha- and beta-subunits to electrophysiologically diverse neuronal K+ currents [1593]
(DTX)
α-dendrotoxin can influence spike latency in Kv1.1 channels. Application of DTX slightly shifted the AP threshold to more hyperpolarized potentials [1595]Dendrotoxin-κ (DTX-κ)a Kv1.1 specific channel blocker was shown to inhibit proliferation of gefitinib-resistant H460 non-small cell lung cancer (NSCLC) cells in vitro and in vivo in a nude mice model[1890]
General anaesthetics
In heterologous expression systems, sevoflurane, isoflurane, and desflurane at subsurgical concentrations potentiated delayed rectifier Kv1 channels at low depolarizing potentials. In mouse thalamic brain slices, sevoflurane inhibited firing frequency and delayed the onset of action potentials in CMT neurons, and ShK-186, a Kv1.3-selective inhibitor, prevented these effects. Our findings demonstrate the exquisite sensitivity of delayed rectifier Kv1 channels to modulation by volatile anesthetics and highlight an arousal suppressing role of Kv1 channels in CMT neurons during the process of anesthesia [1597]
Ankyrin-3
Ankyrin-3 (ANK3) was identified as a binding partner of Kv1.1 and was enriched in isolated distal convoluted tubules as compared to whole kidney. Electrophysiology studies performed in HEK293 cells expressing Kv1.1 showed that ANK3 significantly inhibited Kv1.1-mediated currents (267 compared to 125 pA/pF) for control and ANK3, respectively [1763]
Urotoxin
Scorpion peptide venom urotoxin binds with high affinity to hKv1.2 (IC50 of 160 pM),hKv1.1 and hKv1.3 channels (IC50 = 253 nM and 91 nM respectively)[1889]
(BrMT)2
Non-peptidic snail toxin 6-bromo-2-mercaptotryptamine dimer (BrMT)2 and its derivatives were reported to slow Kv1.1 channel activation [1896]
Hydrogensulfate
Nociceptive processing in trigeminal ganglion (TG) neurons involves endogenous H2S generating enzyme (CBS) co-localization with Kv1.1 and Kv1.4. Application of NaHS, an H2S donor, supresses IK density with impact on neuronal excitability[1893]
Sensitisation to cytotoxins
Tumour cells expressing Kv1.1 or Kv1.3 are more sensitive to cytotoxins (staurosporine, C2-ceramide, cisplatin and clofazimine)[1899]
Phosphatidylinositol-4,5-bisphosphate (PIP2)
Kv1.1/Kv1.2 heteromeric channels in spiral ganglion neurons are positively regulated by phosphatidylinositol-4,5-bisphosphate (PIP2), a possible intrinsic control of rapid spike adaption in the auditory nerve [1914]
References
Interaction of syntaxin with a single Kv1.1 channel: a possible mechanism for modulating neuronal excitability.
Pflugers Arch.,
2007
Jun
, 454 (477-94).
Chi XX
et al.
Manipulation of the potassium channel Kv1.1 and its effect on neuronal excitability in rat sensory neurons.
J. Neurophysiol.,
2007
Nov
, 98 (2683-92).
Christie MJ
et al.
Expression of a cloned rat brain potassium channel in Xenopus oocytes.
Science,
1989
Apr
14
, 244 (221-4).
Lorincz A
et al.
Cell-type-dependent molecular composition of the axon initial segment.
J. Neurosci.,
2008
Dec
31
, 28 (14329-40).
Karcz A
et al.
Low-voltage activated Kv1.1 subunits are crucial for the processing of sound source location in the lateral superior olive in mice.
,
2011
Jan
10
, ().
Glasscock E
et al.
Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy.
J. Neurosci.,
2010
Apr
14
, 30 (5167-75).
Streit AK
et al.
RNA editing of Kv1.1 channels may account for reduced ictogenic potential of 4-aminopyridine in chronic epileptic rats.
,
2011
Mar
3
, ().
Tomlinson SE
et al.
Nerve excitability studies characterize KV1.1 fast potassium channel dysfunction in patients with episodic ataxia type 1.
Brain,
2010
Dec
, 133 (3530-40).
Decher N
et al.
RNA editing modulates the binding of drugs and highly unsaturated fatty acids to the open pore of Kv potassium channels.
EMBO J.,
2010
Jul
7
, 29 (2101-13).
Prüss H
et al.
Age-dependent axonal expression of potassium channel proteins during development in mouse hippocampus.
,
2009
Dec
12
, ().
Bähring R
et al.
Differential modulation of Kv1 channel-mediated currents by co-expression of Kvbeta3 subunit in a mammalian cell-line.
Mol. Membr. Biol.,
2004 Jan-Feb
, 21 (19-25).
Gubitosi-Klug RA
et al.
The human Kv1.1 channel is palmitoylated, modulating voltage sensing: Identification of a palmitoylation consensus sequence.
Proc. Natl. Acad. Sci. U.S.A.,
2005
Apr
26
, 102 (5964-8).
Ogawa Y
et al.
Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2.
J. Neurosci.,
2008
May
28
, 28 (5731-9).
Scott VE
et al.
Antibodies specific for distinct Kv subunits unveil a heterooligomeric basis for subtypes of alpha-dendrotoxin-sensitive K+ channels in bovine brain.
Biochemistry,
1994
Feb
22
, 33 (1617-23).
Inda MC
et al.
Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells.
Proc. Natl. Acad. Sci. U.S.A.,
2006
Feb
21
, 103 (2920-5).
Manganas LN
et al.
Subunit composition determines Kv1 potassium channel surface expression.
J. Biol. Chem.,
2000
Sep
22
, 275 (29685-93).
Wang H
et al.
Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons.
Nature,
1993
Sep
2
, 365 (75-9).
Rho JM
et al.
Developmental seizure susceptibility of kv1.1 potassium channel knockout mice.
Dev. Neurosci.,
1999
Nov
, 21 (320-7).
Doyle DA
et al.
The structure of the potassium channel: molecular basis of K+ conduction and selectivity.
Science,
1998
Apr
3
, 280 (69-77).
Liu HL
et al.
Molecular docking of the scorpion toxin Tc1 to the structural model of the voltage-gated potassium channel Kv1.1 from human Homo sapiens.
J. Biomol. Struct. Dyn.,
2004
Apr
, 21 (639-50).
Baumann A
et al.
Structure of the voltage-dependent potassium channel is highly conserved from Drosophila to vertebrate central nervous systems.
EMBO J.,
1988
Aug
, 7 (2457-63).
Brew HM
et al.
Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1.
J. Physiol. (Lond.),
2003
Apr
1
, 548 (1-20).
Jenkins PM
et al.
Subunit-dependent axonal trafficking of distinct alpha heteromeric potassium channel complexes.
J. Neurosci.,
2011
Sep
14
, 31 (13224-35).
Robbins CA
et al.
Kv1.1 and Kv1.2: Similar channels, different seizure models.
Epilepsia,
2012
Jun
, 53 Suppl 1 (134-41).
Gubitosi-Klug RA
et al.
Fatty acid ethyl esters, nonoxidative metabolites of ethanol, accelerate the kinetics of activation of the human brain delayed rectifier K+ channel, Kv1.1.
J. Biol. Chem.,
1996
Dec
20
, 271 (32519-22).
Wickenden AD
Potassium channels as anti-epileptic drug targets.
Neuropharmacology,
2002
Dec
, 43 (1055-60).
Rhodes KJ
et al.
Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes.
J. Neurosci.,
1997
Nov
1
, 17 (8246-58).
Tytgat J
et al.
Effect of fluoxetine on a neuronal, voltage-dependent potassium channel (Kv1.1).
Br. J. Pharmacol.,
1997
Dec
, 122 (1417-24).
Kirchheim F
et al.
Regulation of action potential delays via voltage-gated potassium Kv1.1 channels in dentate granule cells during hippocampal epilepsy.
Front Cell Neurosci,
2013
, 7 (248).
Al-Sabi A
et al.
Arrangement of Kv1 alpha subunits dictates sensitivity to tetraethylammonium.
J. Gen. Physiol.,
2010
Sep
, 136 (273-82).
Lioudyno MI
et al.
Shaker-related potassium channels in the central medial nucleus of the thalamus are important molecular targets for arousal suppression by volatile general anesthetics.
J. Neurosci.,
2013
Oct
9
, 33 (16310-22).
Al-Sabi A
et al.
Pharmacological characteristics of Kv1.1- and Kv1.2-containing channels are influenced by the stoichiometry and positioning of their α subunits.
Biochem. J.,
2013
Aug
15
, 454 (101-8).
Jan LY
et al.
Voltage-gated potassium channels and the diversity of electrical signalling.
J. Physiol. (Lond.),
2012
Jun
1
, 590 (2591-9).
Punke MA
et al.
Amitriptyline is a potent blocker of human Kv1.1 and Kv7.2/7.3 channels.
Anesth. Analg.,
2007
May
, 104 (1256-64, tables of contents).
San-Cristobal P
et al.
Ankyrin-3 is a novel binding partner of the voltage-gated potassium channel Kv1.1 implicated in renal magnesium handling.
Kidney Int.,
2013
Jul
31
, ().
Lallet-Daher H
et al.
Potassium channel KCNA1 modulates oncogene-induced senescence and transformation.
Cancer Res.,
2013
Aug
15
, 73 (5253-65).
Hao J
et al.
Kv1.1 channels act as mechanical brake in the senses of touch and pain.
Neuron,
2013
Mar
6
, 77 (899-914).
Simeone TA
et al.
Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices.
Neurobiol. Dis.,
2013
Jun
, 54 (68-81).
Zhu J
et al.
Characterization of the Kv1.1 I262T and S342I mutations associated with episodic ataxia 1 with distinct phenotypes.
Arch. Biochem. Biophys.,
2012
Aug
15
, 524 (99-105).
Allen PD
et al.
Kcna1 gene deletion lowers the behavioral sensitivity of mice to small changes in sound location and increases asynchronous brainstem auditory evoked potentials but does not affect hearing thresholds.
J. Neurosci.,
2012
Feb
15
, 32 (2538-43).
van der Wijst J
et al.
Functional analysis of the Kv1.1 N255D mutation associated with autosomal dominant hypomagnesemia.
J. Biol. Chem.,
2010
Jan
1
, 285 (171-8).
Clark E
et al.
Kv1.1 takes a deTOR from the axon to the dendrite.
Neuron,
2006
Nov
9
, 52 (399-401).
Beraud E
et al.
Block of neural Kv1.1 potassium channels for neuroinflammatory disease therapy.
Ann. Neurol.,
2006
Nov
, 60 (586-96).
Gittelman JX
et al.
Kv1.1-containing channels are critical for temporal precision during spike initiation.
J. Neurophysiol.,
2006
Sep
, 96 (1203-14).
Sutachan JJ
et al.
Effects of Kv1.1 channel glycosylation on C-type inactivation and simulated action potentials.
Brain Res.,
2005
Oct
5
, 1058 (30-43).
Watanabe I
et al.
Glycosylation affects rat Kv1.1 potassium channel gating by a combined surface potential and cooperative subunit interaction mechanism.
J. Physiol. (Lond.),
2003
Jul
1
, 550 (51-66).
Petitjean D
et al.
A Disease Mutation Causing Episodic Ataxia Type I in the S1 Links Directly to the Voltage Sensor and the Selectivity Filter in Kv Channels.
J. Neurosci.,
2015
Sep
2
, 35 (12198-206).
Glaudemans B
et al.
A missense mutation in the Kv1.1 voltage-gated potassium channel-encoding gene KCNA1 is linked to human autosomal dominant hypomagnesemia.
J. Clin. Invest.,
2009
Apr
, 119 (936-42).
Imbrici P
et al.
Episodic ataxia type 1 mutations affect fast inactivation of K+ channels by a reduction in either subunit surface expression or affinity for inactivation domain.
Am. J. Physiol., Cell Physiol.,
2011
Jun
, 300 (C1314-22).
Ishida S
et al.
Kcna1-mutant rats dominantly display myokymia, neuromyotonia and spontaneous epileptic seizures.
Brain Res.,
2012
Jan
30
, 1435 (154-66).
Simeone TA
et al.
In vivo ketogenic diet treatment attenuates pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices from epileptic Kv 1.1α knockout mice.
Epilepsia,
2014
May
, 55 (e44-9).
Luna-Ramírez K
et al.
Structure, molecular modeling, and function of the novel potassium channel blocker urotoxin isolated from the venom of the Australian scorpion Urodacus yaschenkoi.
Mol. Pharmacol.,
2014
Jul
, 86 (28-41).
Jeon WI
et al.
Effects of voltage-gated K+ channel blockers in gefitinib-resistant H460 non-small cell lung cancer cells.
Anticancer Res.,
2012
Dec
, 32 (5279-84).
Kurotani T
et al.
Pyramidal neurons in the superficial layers of rat retrosplenial cortex exhibit a late-spiking firing property.
Brain Struct Funct,
2013
Jan
, 218 (239-54).
Brewster DL
et al.
Expression of the voltage-gated potassium channel subunit Kv1.1 in embryonic zebrafish Mauthner cells.
Neurosci. Lett.,
2013
Feb
28
, 539 (54-9).
Feng X
et al.
Hydrogen sulfide increases excitability through suppression of sustained potassium channel currents of rat trigeminal ganglion neurons.
Mol Pain,
2013
, 9 (4).
Sosanya NM
et al.
Degradation of high affinity HuD targets releases Kv1.1 mRNA from miR-129 repression by mTORC1.
J. Cell Biol.,
2013
Jul
8
, 202 (53-69).
Krestel H
et al.
Differences between RNA and DNA due to RNA editing in temporal lobe epilepsy.
Neurobiol. Dis.,
2013
Aug
, 56 (66-73).
Gao D
et al.
Synthesis of the non-peptidic snail toxin 6-bromo-2-mercaptotryptamine dimer (BrMT)2, its lower and higher thio homologs and their ability to modulate potassium ion channels.
Bioorg. Med. Chem. Lett.,
2013
Oct
15
, 23 (5503-6).
Tomlinson SE
et al.
Clinical, genetic, neurophysiological and functional study of new mutations in episodic ataxia type 1.
J. Neurol. Neurosurg. Psychiatr.,
2013
Jan
24
, ().
Streit AK
et al.
RNA editing in the central cavity as a mechanism to regulate surface expression of the voltage-gated potassium channel Kv1.1.
J. Biol. Chem.,
2014
Sep
26
, 289 (26762-71).
Leanza L
et al.
Correlation between potassium channel expression and sensitivity to drug-induced cell death in tumor cell lines.
Curr. Pharm. Des.,
2013
May
16
, ().
Jones KS
et al.
Neonatal NMDA receptor blockade disrupts spike timing and glutamatergic synapses in fast spiking interneurons in a NMDA receptor hypofunction model of schizophrenia.
PLoS ONE,
2014
, 9 (e109303).
Wolfram V
et al.
The transcription factors islet and Lim3 combinatorially regulate ion channel gene expression.
J. Neurosci.,
2014
Feb
12
, 34 (2538-43).
Watanabe T
et al.
Coexpression of auxiliary Kvβ2 subunits with Kv1.1 channels is required for developmental acquisition of unique firing properties of zebrafish Mauthner cells.
J. Neurophysiol.,
2013
Dec
11
, ().
Heeroma JH
et al.
Episodic ataxia type 1 mutations differentially affect neuronal excitability and transmitter release.
Dis Model Mech,
2009 Nov-Dec
, 2 (612-9).
Sosanya NM
et al.
Rapamycin reveals an mTOR-independent repression of Kv1.1 expression during epileptogenesis.
Neurobiol. Dis.,
2015
Jan
, 73 (96-105).
Klassen TL
et al.
High-resolution molecular genomic autopsy reveals complex sudden unexpected death in epilepsy risk profile.
Epilepsia,
2013
Dec
24
, ().
Graves TD
et al.
Episodic ataxia type 1: clinical characterization, quality of life and genotype-phenotype correlation.
Brain,
2014
Apr
, 137 (1009-18).
Lassche S
et al.
A novel KCNA1 mutation causing episodic ataxia type I.
Muscle Nerve,
2014
Mar
18
, ().
Moore BM
et al.
The Kv1.1 null mouse, a model of sudden unexpected death in epilepsy (SUDEP).
Epilepsia,
2014
Nov
, 55 (1808-16).
Kuba H
et al.
Redistribution of Kv1 and Kv7 enhances neuronal excitability during structural axon initial segment plasticity.
Nat Commun,
2015
, 6 (8815).
Bialowas A
et al.
Analog modulation of spike-evoked transmission in CA3 circuits is determined by axonal Kv1.1 channels in a time-dependent manner.
Eur. J. Neurosci.,
2015
Feb
, 41 (293-304).
Meyer SA
et al.
Expression of vimentin by cultured astroglia and oligodendroglia.
J. Neurosci. Res.,
1989
Oct
, 24 (251-9).
Aiba I
et al.
Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models.
Sci Transl Med,
2015
Apr
8
, 7 (282ra46).
Smith KE
et al.
Phosphoinositide Modulation of Heteromeric Kv1 Channels Adjusts Output of Spiral Ganglion Neurons from Hearing Mice.
J. Neurosci.,
2015
Aug
12
, 35 (11221-32).
Kole MJ
et al.
Selective Loss of Presynaptic Potassium Channel Clusters at the Cerebellar Basket Cell Terminal Pinceau in Adam11 Mutants Reveals Their Role in Ephaptic Control of Purkinje Cell Firing.
J. Neurosci.,
2015
Aug
12
, 35 (11433-44).
Swain SM
et al.
Ca(2+)/calmodulin regulates Kvβ1.1-mediated inactivation of voltage-gated K(+) channels.
Sci Rep,
2015
, 5 (15509).
Wright S
et al.
Seizure phenotypes, periodicity, and sleep-wake pattern of seizures in Kcna-1 null mice.
Epilepsy Behav,
2015
Dec
24
, 55 (24-29).
D'Adamo MC
et al.
Novel phenotype associated with a mutation in the KCNA1(Kv1.1) gene.
Front Physiol,
2014
, 5 (525).
Karcz A
et al.
Auditory deficits of Kcna1 deletion are similar to those of a monaural hearing impairment.
Hear. Res.,
2015
Mar
, 321 (45-51).
Glasscock E
et al.
Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation.
Basic Res. Cardiol.,
2015
Sep
, 110 (505).
Gautier NM
et al.
Spontaneous seizures in Kcna1-null mice lacking voltage-gated Kv1.1 channels activate Fos expression in select limbic circuits.
J. Neurochem.,
2015
Oct
, 135 (157-64).
Kuzmenkov AI
et al.
Variability of Potassium Channel Blockers in Mesobuthus eupeus Scorpion Venom with Focus on Kv1.1: AN INTEGRATED TRANSCRIPTOMIC AND PROTEOMIC STUDY.
J. Biol. Chem.,
2015
May
8
, 290 (12195-209).
Contributors: Rajnish Ranjan, Michael Schartner, Erika Borcel, Nitin Khanna, Katherine Johnston
To cite this page: [Contributors] Channelpedia https://channelpedia.epfl.ch/wikipages/1/ , accessed on 2025 Nov 11